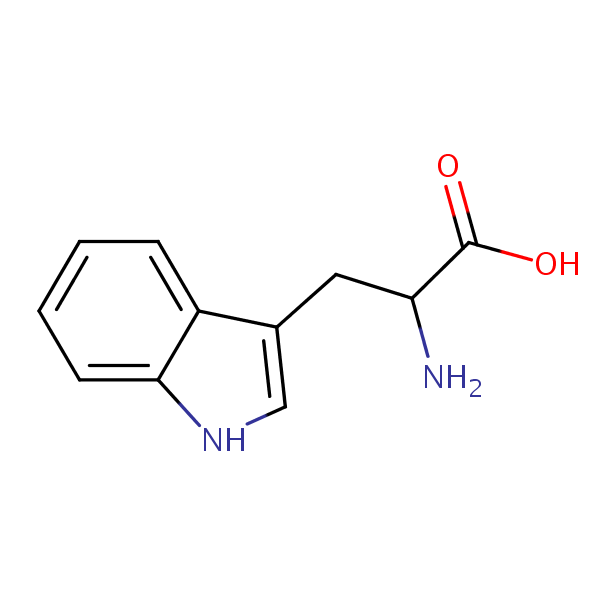
| CAS Number | 54-12-6 |
|---|---|
| Molecular Formula | C11H12N2O2 |
| Molecular Weight | 204.230 |
| InChI Key | QIVBCDIJIAJPQS-UHFFFAOYSA-N |
| LogP | -1.05 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Tryptophan
July 8, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Tryptophan check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

HPLC Method for Separation of a Mixture of Tryptophan and its Catabolites on Primesep 100 Column
October 3, 2023
High Performance Liquid Chromatography (HPLC) Method for Analysis of Mixture of Tryptophan and its Catabolites on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
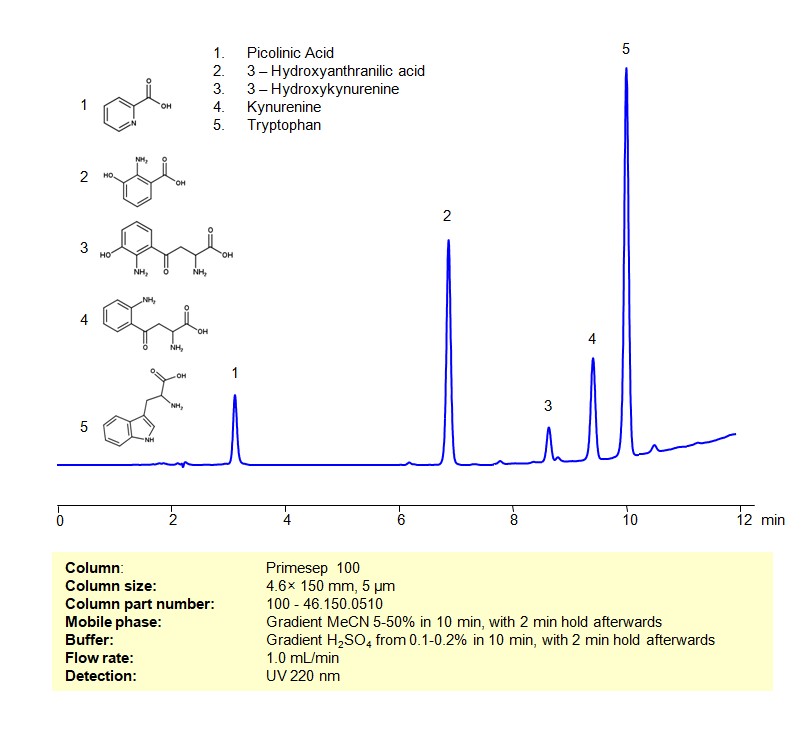
Tryptophan and its catabolites participate in several biological pathways, having roles in protein synthesis, serving as precursors to bioactive molecules, and influencing several physiological processes. Here’s an overview considering a mixture of tryptophan and its catabolites:
Tryptophan:
- Essential Amino Acid: Tryptophan is a precursor to several important compounds, including serotonin and melatonin.
- In Protein Synthesis: Incorporated into proteins during protein synthesis.
Catabolites:
1. Serotonin:
- Neurotransmitter: Regulates mood, appetite, and sleep, among other functions.
- Derivative: Melatonin, which regulates the sleep-wake cycle.
2. Kynurenine Pathway (Major catabolic pathway of tryptophan):
- Kynurenine: An intermediate and precursor to several bioactive compounds.
- Kynurenic Acid: An NMDA receptor antagonist, believed to have neuroprotective effects.
- Xanthurenic Acid: Its physiological roles are still being explored, but it’s often studied for its relation to diabetes and neurological conditions.
- 3-Hydroxykynurenine: Can generate reactive oxygen species, potentially contributing to cellular stress.
- Quinolinic Acid: A neuroactive metabolite that can act as an NMDA receptor agonist.
3. Indoleamine 2,3-dioxygenase (IDO) Pathway:
- Tryptophan can be degraded into several catabolites via the IDO pathway, influencing immune response and cell proliferation.
.
Tryptophan and its Catabolites can be retained, separated and analyzed on a Primesep 100 mixed-mode stationary phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected using UV at 220 nm.
High Performance Liquid Chromatography (HPLC) Method for Analyses of Mixture of Tryptophan and its Catabolites
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN 5-50% in 10 min, with 2 min hold afterwards |
| Buffer | Gradient H2SO4 from 0.1-0.2% in 10 min, with 2 min hold afterwards |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220 nm |
Description
| Class of Compounds | Essential Amino Acid Tryptophan and its Catabolites |
| Analyzing Compounds | Tryptophan, Picolinic Acid, Kynurenine, 3-Hydroxykynurenine, 3-Hydroxyanthranilic acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
3-Hydroxykynurenine
Kynurenine
Picolinic Acid
Tryptophan

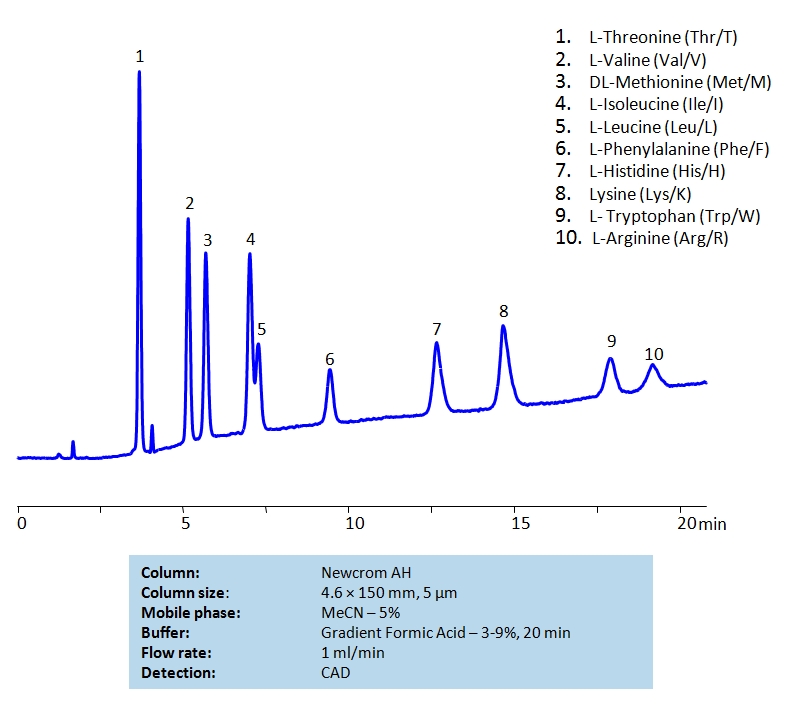
HPLC Separation of Mixture of Nine Essential Amino acids and Arginine on Newcrom AH Column
September 25, 2020
HPLC Method for L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine.
Essential amino acids cannot be made by the body. As a result, they must come from food.
The 9 essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
L-Threonine is an essential amino acid with the chemical formula C4H9NO3. It cannot be produced within the body and must be obtained through consuming it. It’s found in many protein-rich foods, including but not limited to eggs, meat, dairy, legumes, and seeds. It is necessary in the body as a building block of protein like collagen and elastin. The two proteins are crucial for skin, hair, and connective issue.
L-Valine is an essential amino acid with the chemical formula C5H11NO2. It cannot be produced within the body and must be obtained through consuming it. It’s found in foods including but not limited to nuts, legumes, whole grains, and seeds. It is especially beneficial for athletes. It is important for muscle repair, growth, and energy regulation.
DL-Methionine is an essential amino acid with the chemical formula C5H11NO2S. It cannot be produced within the body and must be obtained through consuming it. It is required for protein synthesis. It also helps build and repair tissue including, but not limited to, skin, hair, muscles, and nails. In a veterinary context, DL-Methionine is used to address bladder issues in dogs.
L-Isoleucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It is a building block of protein that are essential for muscle growth, repair, and other bodily functions. It also helps regulate blood sugar levels and supports the immune system. It is found in foods like meat, fish, eggs, dairy, beans, lentils, nuts, and seeds.
L-Leucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It stimulates production of protein that are essential for muscle building and repair. Meats are the easiest way to get L-Leucine in significant amounts.
L-Phenylalanine is an essential amino acid with the chemical formula C6H9NO2. It cannot be produced within the body and must be obtained through consuming it. It is typically found in high protein foods such as meat, eggs, and fish. Outside of being important for creation of protein, it is also used in treatment for skin disorders and depression.
L-Histidine is an essential amino acid with the chemical formula C9H11N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts.
L-Tryptophan is an essential amino acid with the chemical formula C11H12N2O2. It cannot be produced within the body and must be obtained through consuming it. Like the other essential proteins, it is a building block for protein and muscle tissue, but it is also converted in the body into serotonin, which affects mood. L-Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy.
Lysine is an essential amino acid used in the synthesis of proteins. In biological conditions, it is a basic, charged molecule.
L-Arginine is an essential amino acid used in the synthesis of proteins.
L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine can be retained and analyzed using the Newcrom AH stationary phase column. The analysis utilizes a gradient method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a Formic Acid buffer. Detection is performed using CAD.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 5% |
| Buffer | Gradient Formic Acid – 3-9%, 20 min |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Histidine
Isoleucine
L-Threonine
Leucine
Lysine
Methionine
Phenylalanine
Tryptophan
Valine

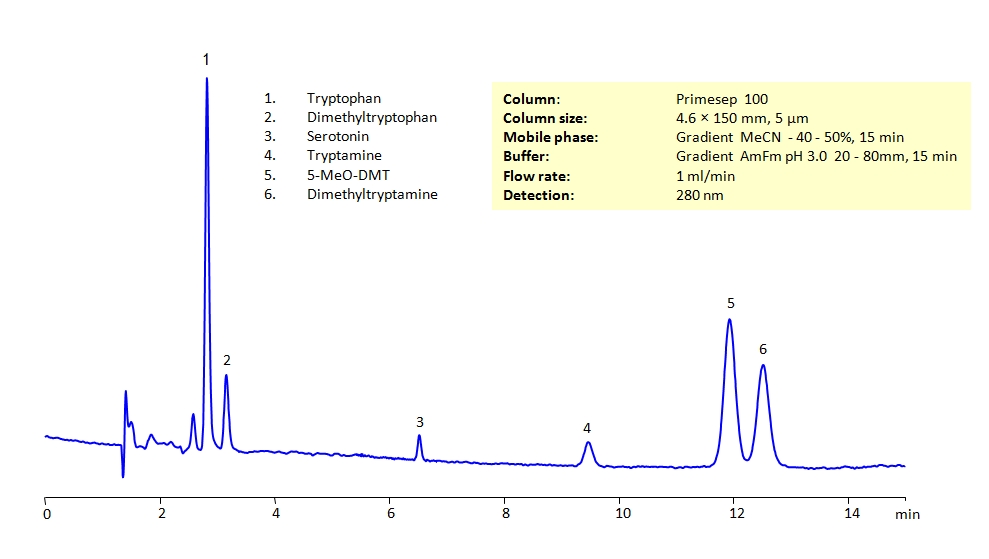
HPLC Separation of Biogenic Amines on Primesep 100 Column
April 30, 2020
HPLC Method for Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan.
Tryptophan is an essential amino acid used in the synthesis of proteins. It cannot be produced by the body and therefore must be consumed through a diet of meats, eggs, dairy, seeds, and nuts. The proteins that it helps synthesize are important for muscle growth and repair. Tryptophan is also converted into serotonin, which is responsible for mood, appetite, and pain reception. It is further down converted into melatonin. Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy. It’s chemical formula is C11H12N2O2. You can find detailed UV spectra of Tryptophan and information about its various lambda maxima by visiting the following link.
Tryptamine is an indolamine derived from tryptophan with the chemical formula C10H12N2. It is naturally found in plants, fungi, and animals. It helps with regulating the activity of dopaminergic, serotonergic, and glutamatergic systems. You can find detailed UV spectra of Tryptamine and information about its various lambda maxima by visiting the following link.
Serotonin is a neurotransmitter derived from tryptophan with the chemical formula C₁₀H₁₂N₂O. Serotonin is a chemical messenger that is crucial for bodily functions. It helps regulate mood and reduce anxiety, digestion, blood clotting, and contributes to the sleep-wake cycle. Low serotonin levels are often associated with a variety of mental illnesses including but not limited to Obsessive Compulsive Disorder (OCD), Post-Traumatic Stress Disorder (PTSD), Depression, and Anxiety.
Dimethyltryptamine is a psychedelic substance derived from tryptamine with the chemical formula C12H16N2. It works as an agonist for Serotonin. It is not approved for medical use. Due to it’s high risk of abuse, it is a Schedule I drug in the United States.
5-MeO-DMT, also known as 5-methoxy-N,N-dimethyltryptamine), O-methylbufotenin, and mebufotenin, is a psychedelic substances derived from tryptamine found in plants and toad secretions. Between 1970s to 1990s, a certain religious sect believed the use of mebufotenin to be sacred to their religious practice. It is currently considered a Schedule I controlled substance in the United States, however.
All the compounds have similar structures and can present difficulties to separation in reverse-phase HPLC. They can be separated using the Primesep 100 mixed-mode column in gradient analysis with acetonitrile (ACN) and water mobile phase with ammonium formate (AmFm) buffer, making the method MS-compatible. The amines can also be UV detected at 280nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 40-50%, 15 min |
| Buffer | Gradient AmFm pH 3.0- 20-80 mM, 15 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm, MS-compatible mobile phase |
| Class of Compounds |
Drug, Hydrophilic, Supplements, Monoamine, Neurotransmitter |
| Analyzing Compounds | Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
N,N-Dimethyltryptamine
N,N-dimethyltryptophan
Serotonin
Tryptamine
Tryptophan

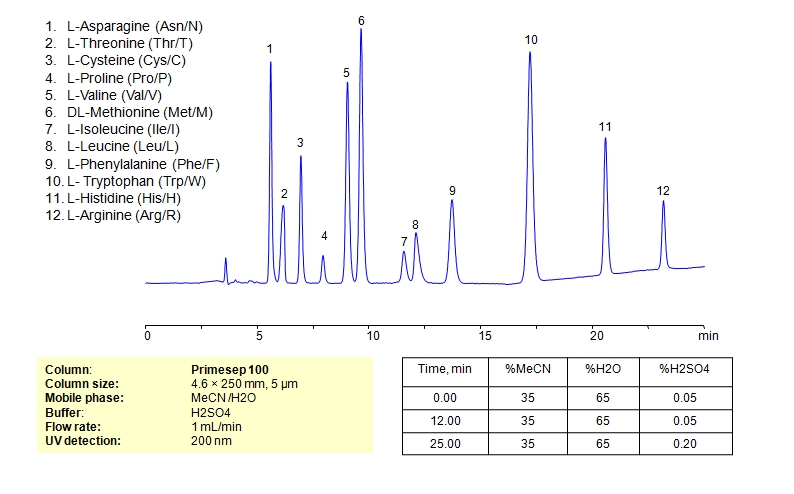
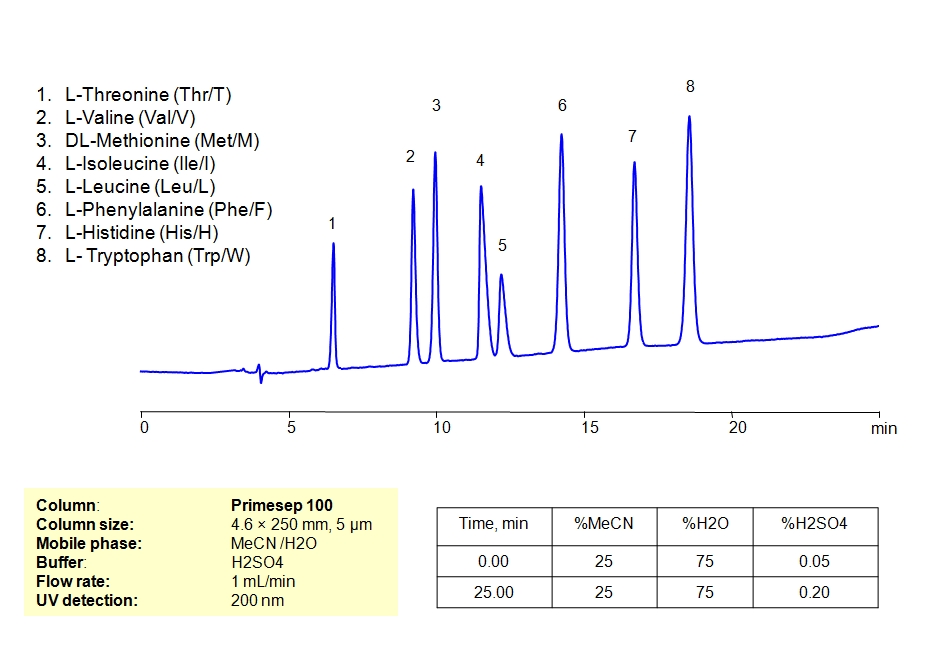
HPLC Separation of Mixture of 12 Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine.
Amino acids are the building blocks of proteins. Based on their dietary requirement, they are classified into essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body in sufficient quantities and must be obtained from the diet. Non-essential amino acids, on the other hand, can be synthesized by the body and are not dependent on dietary intake.
It’s worth noting that while these amino acids are considered “non-essential” for adults under normal circumstances because the body can synthesize them, there are situations where some may become “conditionally essential.” This means that under certain conditions like illness, stress, or trauma, the body might not produce them in sufficient quantities, and dietary intake becomes necessary. Arginine, for instance, is considered conditionally essential, especially during periods of rapid growth, illness, or trauma.
Amino acids can be retained, separeted and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid (H2SO4) as a buffer. This analysis method can be detected in the UV regime at 200 nm.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | H2SO4 0.05% 12 min hold, gradient 0.05-0.20, 13 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Arginine
Asparagine
Cysteine
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Cysteine
L-Methionine
L-Threonine
Leucine
Methionine
Phenylalanine
Proline
Tryptophan
Valine

HPLC Separation of Mixture of Essential Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine.
L-Threonine is an essential amino acid with the chemical formula C4H9NO3. It cannot be produced within the body and must be obtained through consuming it. It’s found in many protein-rich foods, including but not limited to eggs, meat, dairy, legumes, and seeds. It is necessary in the body as a building block of protein like collagen and elastin. The two proteins are crucial for skin, hair, and connective issue.
L-Valine is an essential amino acid with the chemical formula C5H11NO2. It cannot be produced within the body and must be obtained through consuming it. It’s found in foods including but not limited to nuts, legumes, whole grains, and seeds. It is especially beneficial for athletes. It is important for muscle repair, growth, and energy regulation.
DL-Methionine is an essential amino acid with the chemical formula C5H11NO2S. It cannot be produced within the body and must be obtained through consuming it. It is required for protein synthesis. It also helps build and repair tissue including, but not limited to, skin, hair, muscles, and nails. In a veterinary context, DL-Methionine is used to address bladder issues in dogs.
L-Isoleucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It is a building block of protein that are essential for muscle growth, repair, and other bodily functions. It also helps regulate blood sugar levels and supports the immune system. It is found in foods like meat, fish, eggs, dairy, beans, lentils, nuts, and seeds.
L-Leucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It stimulates production of protein that are essential for muscle building and repair. Meats are the easiest way to get L-Leucine in significant amounts.
L-Phenylalanine is an essential amino acid with the chemical formula C6H9NO2. It cannot be produced within the body and must be obtained through consuming it. It is typically found in high protein foods such as meat, eggs, and fish. Outside of being important for creation of protein, it is also used in treatment for skin disorders and depression.
L-Histidine is an essential amino acid with the chemical formula C9H11N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts.
L-Tryptophan is an essential amino acid with the chemical formula C11H12N2O2. It cannot be produced within the body and must be obtained through consuming it. Like the other essential proteins, it is a building block for protein and muscle tissue, but it is also converted in the body into serotonin, which affects mood. L-Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy.
Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | Gradient H2SO4 0.05-0.2% 25 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Histidine hydrochloride monohydrate
L-Isoleucine
L-Methionine
L-Threonine
Methionine
Phenylalanine
Tryptophan
Valine

Generic Screening Method for Complex Mixtures on Primesep 200
October 15, 2015
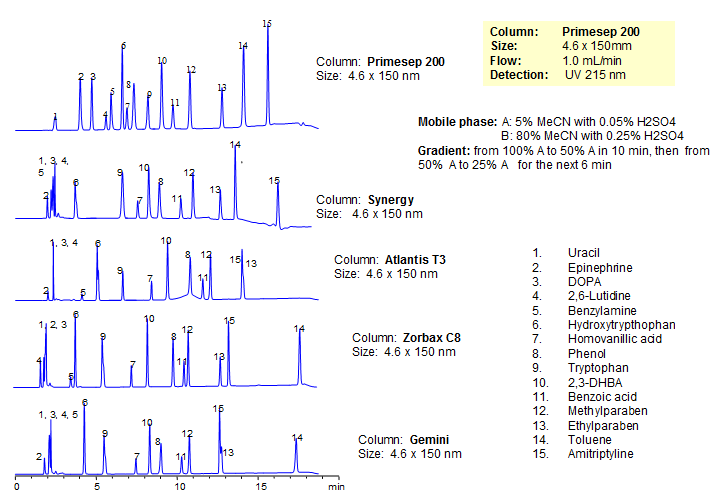
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Separation of Amino Acids on Obelisc R Column
October 4, 2007
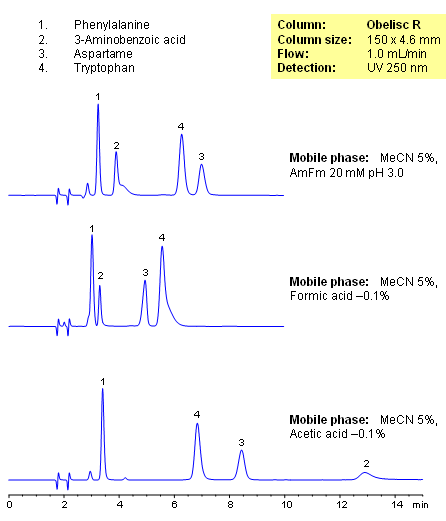
Closely related compounds like amino acids can be separated on an Obelisc R column by various buffers depending on the amount of baseline separation required. By choosing different buffers, the separation between compounds can be adjusted based on application needs, especially those that require low organic concentration in the mobile phase. UV detection at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsAspartame
Phenylalanine
Tryptophan

HPLC Separation of Phenylalanine, Glucosamine, and Tryptophan on Mixed-Mode Column
October 4, 2007
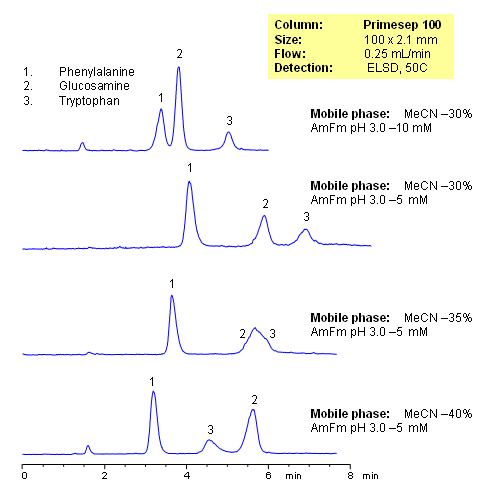
The Separation of Phenylalanine, Glucosamine and Tryptophan demonstrates how the small changes to the organic and/or buffer concentrations in the mobile phase affect the retention of compounds on a mixed-mode column by hydrophobic, ion-exchange and ion-exclusion mechanisms in reverse-phase HPLC chromatography. Evaporative Light Scattering Detector (ELSD) used
| Column | Primesep 100, 2.1×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 0.25 ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Phenylalanine, Glucosamine, Tryptophan |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPhenylalanine
Tryptophan

HPLC Separation of Amino Acids, Bases, Acids, and Neutrals on Obelisc R
March 3, 2007
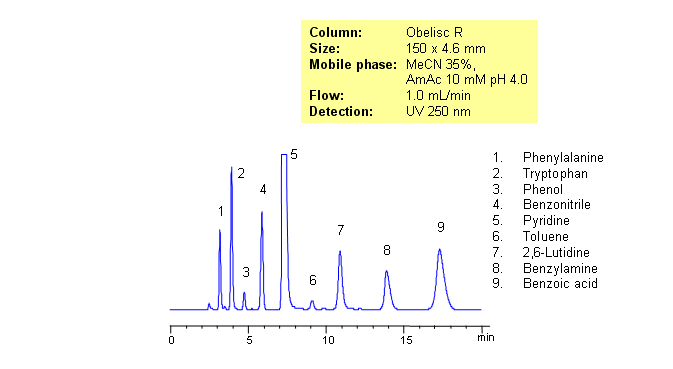
Separating basic, acidic and zwitterionic compounds in one run in reverse-phase HPLC can be very challenging. The methods might require the use of ion-pairing reagents and complex gradients that can make MS-compatibility difficult. Obelisc R column which has both positive and negative ion-pairs embedded in the stationary phase allows for fine tuning and separation of a wide range of compounds with different ionic properties. Acids, bases, amino acids and neutral compounds were separated isocratically in one run using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Acetate (AmAc) buffer. Can also be UV detected at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | AmAc 10 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Bases, Neutral, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Phenol
Phenylalanine
Pyridine
Toluene
Tryptophan