|
Buffer Preparation FAQs
What does “buffer molarity” mean?
Is it practical to adjust the pH of a mixture of water with acetonitrile?
When mobile phase contains 0.1% of TFA or any other acid, how do you prepare it?
What does “buffer molarity” mean?
In all of the SIELC applications, the buffer molarity (usually in mM) is the number of moles of a cation portion of the buffer excluding concentration of H + ions.
For example, 1 L of ammonium acetate (buffer 20, mM pH 4.0) will be prepared the following way: 100 mmol of ammonium acetate salt is dissolved in a little less than 1000 mL of water, the acidity of the solution is adjusted to pH=4.0 by (adding) acetic acid and total volume is adjusted to 1000 mL with water. In this example, the molarity of the buffer is based on molarity of ammonia. The concentration of the acetic acid is unknown. Take 200mL of your prepared solution and adjust it up to 1000mL with your mobile phase.
back to top
Is it practical to adjust the pH of a mixture of water with acetonitrile?
We do not recommend that you do this because the pH meters are calibrated by aqueous pH standards. The value of the pH in an organic/water solution can be different from the (actual) real pH value. The problem can be even more complex if different types of pH electrodes are used by a different lab. In order to get a mobile phase with both MeCN and water with buffer at certain pH, we prepare a stock solution of concentrated buffer, adjust the pH, and then diluted it with water and MeCN to desirable molarity and MeCN concentration (until a desirable molarity and MeCN concentration are reached). For example, a 25 mM buffer of ammonium formate, pH 3.5, with 50% MeCN would be prepared from 25% of 100 mM AmFm buffer pH 3.5 plus 25% water plus 50% MeCN.
back to top
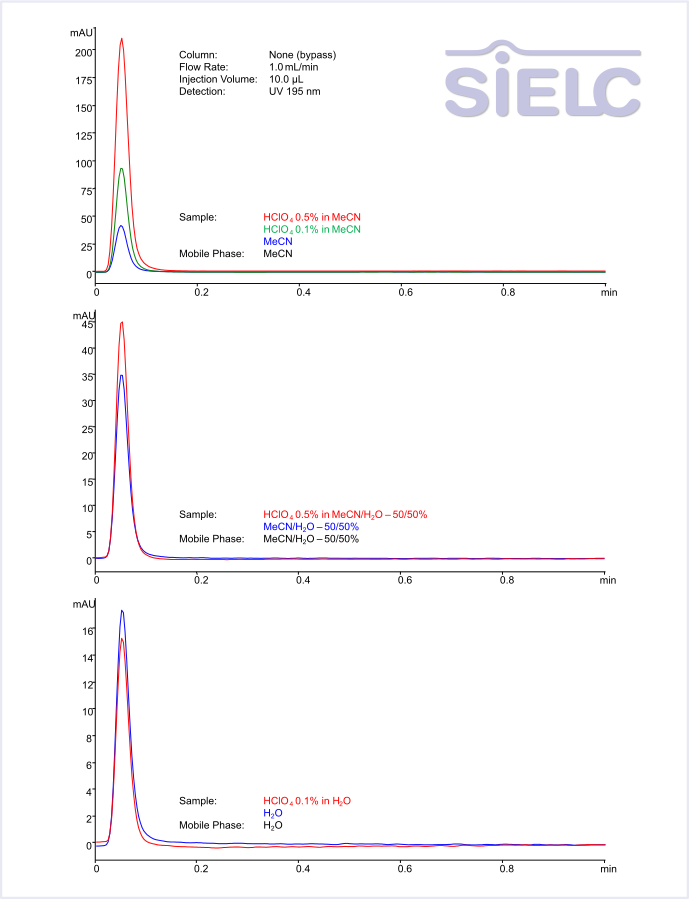
When mobile phase contains 0.1% of TFA or any other acid, how do you prepare it?
We typically use 1% solution of acids in water as a stock solution. When we are making 1% stock solution of TFA, which is a very volatile acid, we prefer to weight the acid rather than use volumetric pipets. TFA density is 1.49. To make a 1 L of 1% TFA solution, we dissolved 10 g of TFA in water to the 1000 mL of solution. Click here to find the density of acids useful in HPLC.
back to top
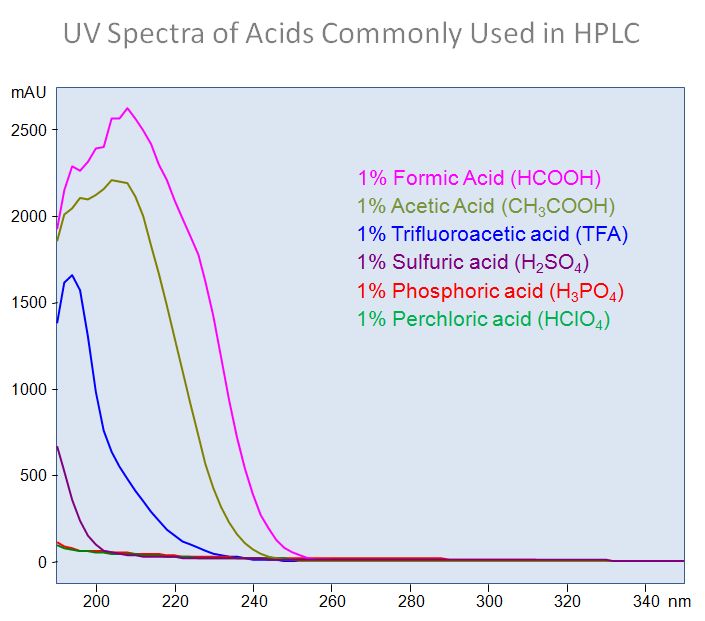
What is UV cut-off of common buffers?
The lowest wavelength a buffer can be used depends on buffer concentration and in most part UV spectra of acidic component of the buffer. Especially if you work in acidic buffer pH.
Spectra of common acids useful in HPLC analysis presented on picture below.
|