HPLC Retention of Polar Compounds and Separation of Polar CompoundsThe reverse-phase mode of HPLC is the technique of choice to solve many separation problems. One of the limitations of reverse-phase columns is the lack of retention of highly polar compounds on conventional stationary phases. Traditionally, the ion-pairing reagents have to be employed to achieve the separation of these compounds. In its turn, the use of ion-paring reagents also has its limitations, i.e. artifacts when using gradient elution, incompatibility with Mass Spectrometry (MS), Evaporating Light Scattering Detection (ELSD), preparative chromatography, and more complex mobile phase preparations.
|
|
Obelisc R columnObelisc R has reversed-phase character and can be used in traditional, reversed-phase type applications. Due to the presence of ionic groups and a long hydrophobic chain, Obelisc R offers additional retention and tuning that is not available with traditional reversed-phase columns. Typical mobile phases used with Obelisc columns are based on acetonitrile, water, and the mass spec compatible buffers ammonium formate (pH 3) and ammonium acetate (pH 5). If it is necessary to detect in low UV (<220 nm) then phosphate buffer is recommended. |
|
|
Obelisc R offers a large improvement in the retention of polar compounds over traditional reversed-phase columns. Capacity factors (k’) for 10 polar compounds are plotted for Obelisc R and two common reversed-phase columns. In all cases Obelisc R has the most retention–up to a 10 times more retention. The two reversed-phase columns show little appreciable difference from each other.
|
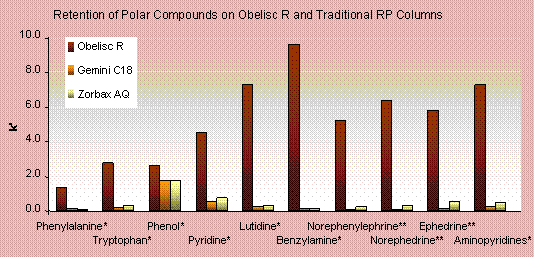 |

© SIELC Technologies. 2002 - 2026
Sign up to our newsletter
Contact
Address: 804 Seton Court, Wheeling, IL USA 60090
Tel: (847) 229-2629 | Fax: (847) 655-6079
Sales, Refund and Returns Policy
Email: mail@sielc.com | Sitemap
Host a Customized Seminar at Your Company
Delivering tailored seminars directly to your team.
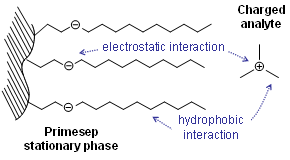
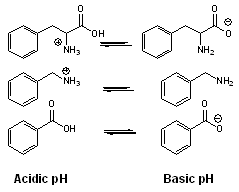 . Therefore, charged analytes are un-retained by hydrophobic interactions alone. Ionizable compounds are much more hydrophobic in their neutral state than their ionized form and can often be retained by reversed-phase in this state. This concept is frequently employed to retain carboxylic acids by reversed-phase under acidic conditions (pH below 3) where they are neutral.
. Therefore, charged analytes are un-retained by hydrophobic interactions alone. Ionizable compounds are much more hydrophobic in their neutral state than their ionized form and can often be retained by reversed-phase in this state. This concept is frequently employed to retain carboxylic acids by reversed-phase under acidic conditions (pH below 3) where they are neutral. 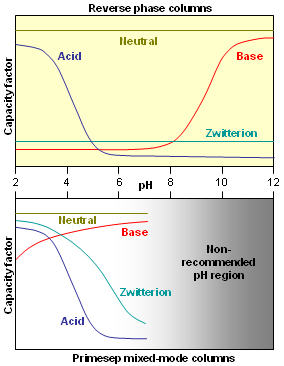 However, for bases, the mobile phase pH must be above 11 for the compounds to be neutral and therefore hydrophobic enough for retention under reversed-phase conditions. Operation under these conditions required special phases with hybrid particles that are stable at a high pH.
However, for bases, the mobile phase pH must be above 11 for the compounds to be neutral and therefore hydrophobic enough for retention under reversed-phase conditions. Operation under these conditions required special phases with hybrid particles that are stable at a high pH.