Cannsep Columns for Cannabinoids
SIELC has developed a special line of Cannsep columns to resolve most of the compounds attributed to cannabis physiological properties. Three columns: Cannsep A, Cannsep B, and Cannsep C resolve all the cannabinoids providing different and significantly orthogonal selectivity.
You can quickly setup your own chemical lab for analysis of marijuana products using our Alltesta Analyzer, Cannsep columns and HPLC methods tailored for this analysis.
Columns are available in all standard dimensions and with particles 5 µm for regular HPLC and 3 µm for UPLC (Ultra-high Pressure Liquid Chromatography).
Cannsep Products
-

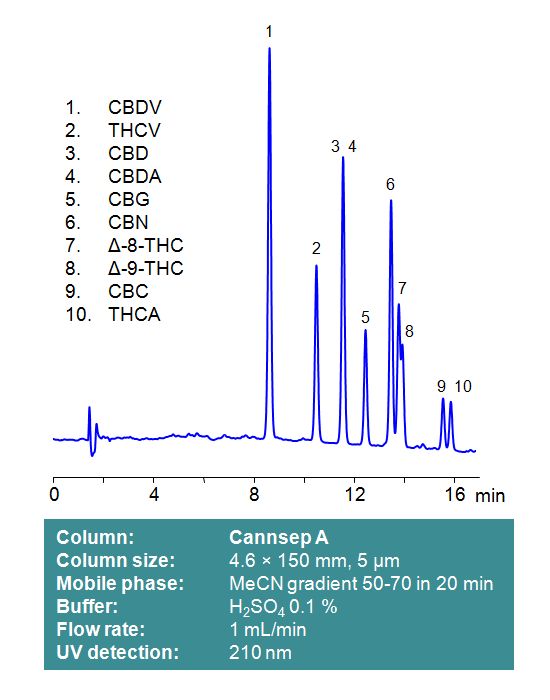
Cannsep A
Cannsep A is a reverse-phase analytical column with embedded strong acidic ion-pairing groups. This stationary phase retains neutral cannabinoids through a reverse-phase mechanism. Acidic cannabinoids, such as Tetrahydrocannabinolic acid (THCA) and Cannabidiolic acid (CBDA), will be subject to ion-exclusion interactions with the negatively charged stationary phase of Cannsep A. Mobile phases can be selected for compatibility with low UV detection, using phosphoric or sulfuric acid as modifiers. Alternatively, LC-MS and preparative chromatography compatible mobile phases can be used with formic acid as a modifier. Select options -

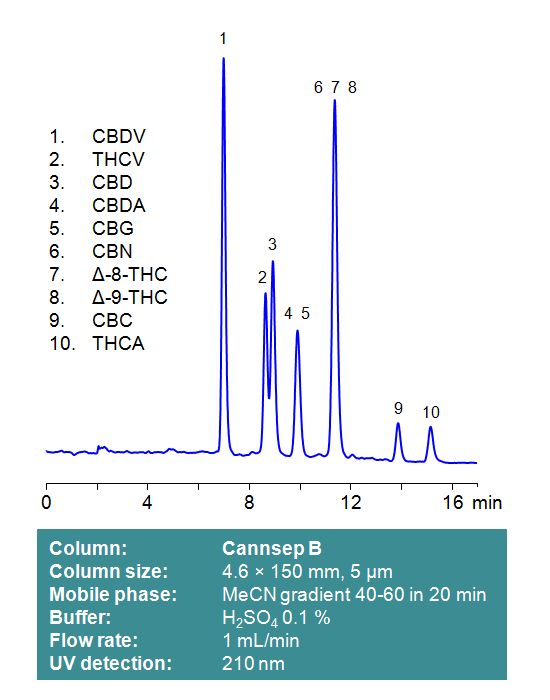
Cannsep B
Cannsep B is a reverse-phase analytical column with embedded strong basic ion-pairing groups. This stationary phase retains neutral cannabinoids through a reverse-phase mechanism. Acidic cannabinoids, such as Tetrahydrocannabinolic acid (THCA) and Cannabidiolic acid (CBDA), will be subject to ion-exchange interactions with the positively charged stationary phase of Cannsep B. Mobile phases can be selected for compatibility with low UV detection, using phosphoric or sulfuric acid as a modifier. Alternatively, LC-MS and preparative chromatography compatible mobile phases can be used with formic acid as an ionic modifier. Select options -

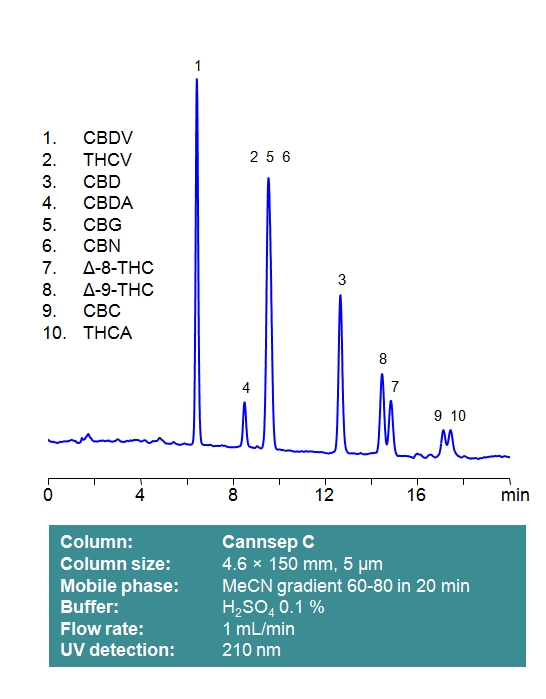
Cannsep C
Cannsep C is a reverse-phase analytical column with a C18 stationary phase and effective end-capping. This stationary phase retains all neutral and acidic cannabinoids through a reverse-phase mechanism. A well-deactivated silica surface and uniform particle distribution provide good peak symmetry and high column efficiency. Mobile phases can be selected for compatibility with low UV detection, using phosphoric or sulfuric acid as a modifier. Alternatively, LC-MS and preparative chromatography compatible mobile phases can be used with formic acid as an ionic modifier. Select options