Chromatography FAQsCan neutral compounds be analyzed on Primesep columns? What is working pH range for Primesep columns? What is the difference among different Primesep phases? Is Primesep silica gel with smaller than 5 um particles available? What is the typical approach to method development? Can I use MeOH instead of MeCN? Are columns with embedded polar groups different from SIELC mixed mode columns? Is there a difference in ionization of molecules in the mobile phase and in the stationary phase? What do the numbers mean in the column name? Is it true that difficult access to silanol can be a problem for obtaining a good peak shape? back to top Yes, every Primesep column is a reverse phase column. Neutral compounds can be analyzed with a water/organic mobile phase in the isocratic or gradient mode. Different Primesep phases offer different selectivity toward neutral compounds, due to difference in polar embedded functional group. back to top Primesep columns are silica based columns. Other than Primesep B, they all have a pH range from 1.5 to 7.0. The recommended working pH range for Primesep B columns is from 1.5 to 4.5 back to top Every phase is different in the degree and polarity of the ion-exchange interaction with analytes. They are all very similar in hydrophobic interaction. back to top Primesep columns offer very high selectivity, and an increase in the efficiency obtained by smaller particles contributes very little to the overall resolution. However, if you still need columns smaller than 5 µm particles, the columns are available in 3 and 2.7 µm format. 10 µm and bigger particle sizes are available for some Primesep phases to be used in preparative chromatography. back to top We recommend starting method development using a short 50 mm column. A high ion-strength mobile phase with a high organic concentration is a good starting point. In many cases, a 50 mm column is sufficient to resolve compounds different in their hydrophobicity or pKa value. If the efficiency of a 50 mm column is not sufficient, a longer column can be used up to 250 mm. See more details on method development protocol in our brochure. back to top We do not recommend using methanol. For most applications MeCN does a better job than MeOH because of the low viscosity and lower UV transparency. There is a danger of esterification of an embedded carboxylic acid group by methanol. In some cases when nitrogen specific detector should be used, another alcohol, such as t-BuOH, can be used instead.
Polar or polarizable groups are significantly different from ionizable groups which we have in every SIELC mixed-mode column. There are a lot of very polar groups in the RP system within the stationary phase and in the mobile phase. Water and silanol groups are examples. So embedded polar groups can not significantly effect the column performance in a reverse phase environment. Ionization is extreme case of polarization, when the separation of charges is equal to infinity. This is a very significant difference with a charge separated only by a few bonds in polar functional groups. Having an ionizable group is a new interaction – electrostatic interaction becomes possible.
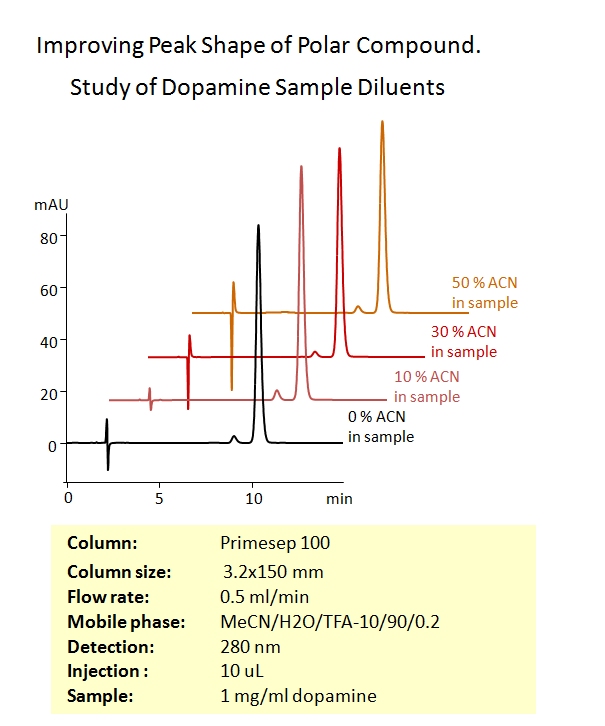
No. When two interactions are involved in retention of a compound, the mobile phase composition can be very different from the mobile phase as long as at least one mode of separation is not disturbed. For example, when a positively charged molecule is retained on the Primesep 100 column, the sample diluent can have any amount of MeCN. The retention will be still non-disturbed due to the insignificant effect of organic concentration on electrostatic interaction. See example.
Pore size (in fact pore diameter) and surface area have nothing to do with pore length. Since typical silica gel has a uniform porous structure (not just pores on the surface region) the pore’s length is in direct proportion to the size of the particles. The UPLC is one example of an attempt to improve column efficiency by going to extra short pores (i.e. extra small particles).
In fact, to do pure ion-exchange separation, the acids need to be in ionized form. However, at a pH below the pKa of the carboxylic acid, the ionization is not significant and the molecules become significantly less polar. In this case they can be retained by the RP mechanism. When molecules end up within a hydrophobic stationary phase layer, the meaning of the pH is different than in aqueous solution, and electrostatic interaction still takes place. This is an example of an RP method with some contribution of IE interaction to improve selectivity.
In the RP system we have two bulk layers: one is a water and MeCN mixture, and the other one is MeCN with CH3(CH2)n-Si-. They are (should be) quite different in solvation properties. If a charged ion or very hydrophilic molecule ends up in the RP stationary phase, it should lose some water shielding. Of course density and the length of the alkyl chain will effect the distribution of both analytes and MeCN between the two layers (SP and MP).
A surfactant or molecule of dual polarity will definitely try to stay on the border of two phases. In the case of Primesep phases, this may change drastically because of electrostatic forces; if they have opposite charges, they can drag the polar head of the surfactant in the SP. This increases retention manyfold.
Your assumption is right for aqueous solutions, but in many cases RP chromatography is
The number in the column name corresponds to the pKa value of the embedded ionizable group. For example, for Primesep 100 the pKa of the embedded carboxylic acid is equal to 1.0. In Primesep 200 ,the pKa = 2.0. For this reason, Primesep C can be called Primesep 350 because the pKa is about 3.5. The letter C in the Primesep C column reflects additional c omplex properties of this stationary phase toward primary and secondary amines in high organic mobile phase.
That is probably not true. At least the difference is not significant in the chromatography time scale. The main limitation is usually diffusion from and in the pores. It is why smaller particle packing always gives higher efficiency per meter than bigger particle columns. They simply have shorter pores. Ionic interaction is generally very strong but it is reduced significantly by the ion-exchange process (competing ions from the mobile phase), so the rate should be close to the diffusion rate. Otherwise, no good chromatography is possible.
Primesep phases have many more ionizable groups for ionic interaction than an RP column has residual silanol groups, especially at an acidic pH. In addition, silica gel has more than one type of silanol group (three types well characterized) with probably a different degree of ionic interaction. So most active silanol groups can be easily overloaded with basic analytes. Good peak shape is common for classical ion-exchange chromatography, so if you have a uniform ion-exchange system and enough sites for interaction, the peak shape shouldn’t be a problem.
This is probably not a correct statement. A sterical problem cannot be observed in electrostatic interaction because this is long force interaction. Oppositely charged ions interact as far as a few hundred angstroms in an aqueous salt solution. This makes ion-exclusion chromatography possible. The real reason for unwanted silanol interaction of basic and specially basic hydrophobic analytes is that acidic silanols can have some degree of ionization, even at a pH of the MP below 2, because they are shielded from the MP by the RP layer. The analytes, while within this layer, can interact with silanol electrostatically because the local pH within the stationary phase layer is not the same as in the MP. Solvated H+ ions have much more affinity to an aqueous MP than to a hydrophobic RP, and as result the pHs of the MP and RP are very different. If the mixture only slightly differs in molecular weight, then you will most likely obtain just one peak. Mobile phase viscosity > |

© SIELC Technologies. 2002 - 2026
Sign up to our newsletter
Contact
Address: 804 Seton Court, Wheeling, IL USA 60090
Tel: (847) 229-2629 | Fax: (847) 655-6079
Sales, Refund and Returns Policy
Email: mail@sielc.com | Sitemap
Host a Customized Seminar at Your Company
Delivering tailored seminars directly to your team.