Normal phase chromatography is a type of liquid chromatography where the stationary phase is more polar than the mobile phase. Fig. 1 shows an application scope of the major types of chromatography techniques for separation of small molecules. The term normal phase used to distinguish this type of chromatography from reverse phase chromatography (this is most common type of analytical separation) where the stationary phase is less polar than the mobile phase. HILIC is a special case of normal phase chromatography. It is used primarily for separation of very polar compounds with a negative log P factor. See picture below.
The term HILIC stands for Hydrophilic Interaction LIquid Chromatography.
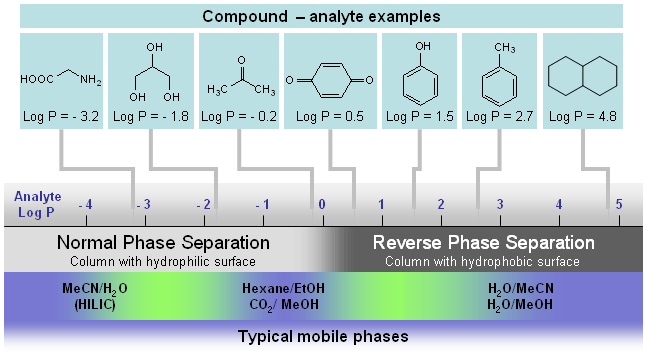
Figure 1.
Graphical representation of different chromatography techniques and their application for separation of molecules of different polarity. Log P is a factor which represents the polar properties of the molecules. It is defined as a logarithm of the distribution coefficient of the compound in the liquid/liquid extraction system of water and octanol. The higher the Log P value, the more hydrophobic the compound is.
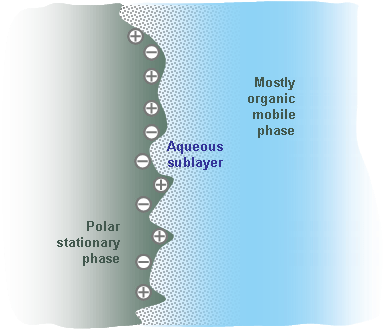 Figure 2. Graphical representation of the micro environment around the surface of the stationary phase in HILIC chromatography. |
The typical mobile phase for HILIC chromatography includes MeCN with a small water content. It is commonly believed that in HILIC, the mobile phase forms a water rich layer on the surface of the polar stationary phase creating a liquid/liquid extraction system (Fig. 2). The analyte is distributed between the water rich stationary layer and the mobile phase with low water contents. More polar compounds will have a higher affinity (stronger polar interaction) to the stationary aqueous layer than less polar compounds. Thus, the separation based on compound polarity will take place.
There are several types of columns available for HILIC separation. Their retention profile is not much different since most of the separation occurs between two layers: the water rich layer on a stationary phase and a mainly organic mobile phase. The polar surface of the stationary phase usually forms by ionized or very polar groups of solid material. The column surface itself does not participate in this type of interaction; it just provides a polar environment which forms a water sub-layer. Primesep N column is one of common HILIC column that can be used for traditional HILIC type separation. An exception is the case when the surface and analytes are engaged in electrostatic interaction. |
The Obelisc N column is designed for any type of normal phase separation including HILIC. This column is different from other types of HILIC columns because of its special surface chemistry.
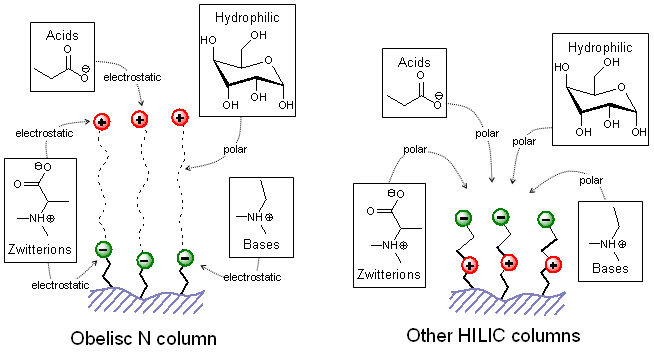
Figure 3.
Type of interactions available with Obelisc N column vs. other HILIC columns.
In all types of HILIC columns the polar surface is formed by closely spaced and oppositely charged functional groups or by silica itself. This configuration precludes the stationary phase to interact with analytes by electrostatic forces (except a silanol interaction). The Obelisc N chemistry offers significant distance between positively charged functional groups and negatively charged functional groups so they both are available for an electrostatic interaction. As result, the retention of charged molecules is significantly enhanced on Obelisc N column. This column can be operated in wide range of organic modifier concentrations from 0% to 100% of MeCN. It is a wider range than in any other HILIC column. Additionally, Obelisc N column can be operated in MeOH based mobile phases, while all other HILIC columns failed to produce any significant retention. This is important when the nitrogen specific detector requires by a method.
Applications examples of Obelisc N column:
Other HILIC applications examples:
| HPLC Separation of Aromatic Diamine Disulfate | |
| HPLC Separation of Heparines and Pentosan | |
| HPLC Separation of Morpholino Sulfates | |
| HPLC Separation of Uridine and Uracil | |
