| CAS Number | 61-54-1 |
|---|---|
| Molecular Formula | C10H12N2 |
| Molecular Weight | 160.221 |
| InChI Key | APJYDQYYACXCRM-UHFFFAOYSA-N |
| LogP | 1.55 |
| Synonyms |
|
Applications:
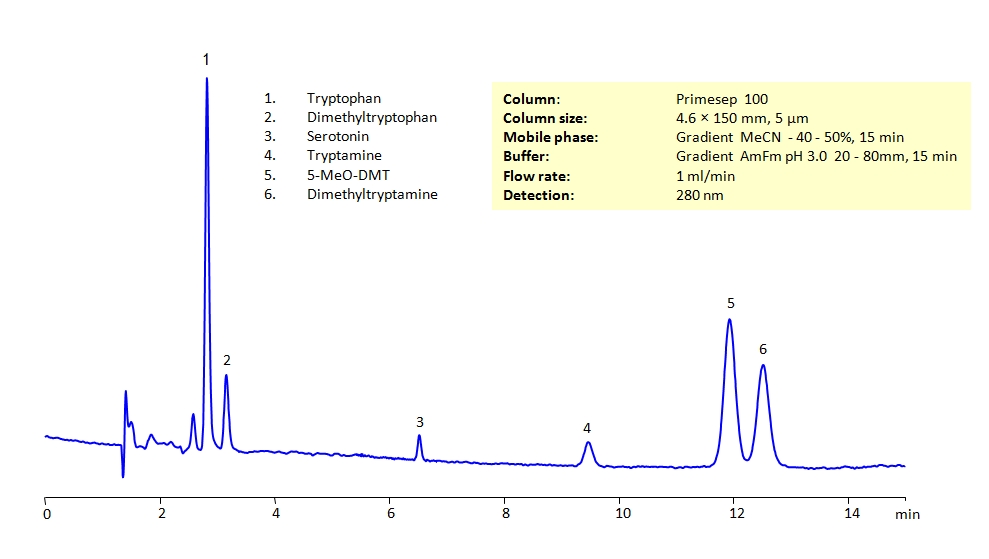
HPLC Separation of Biogenic Amines on Primesep 100 Column
April 30, 2020
HPLC Method for Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan.
Tryptophan is an essential amino acid used in the synthesis of proteins. It cannot be produced by the body and therefore must be consumed through a diet of meats, eggs, dairy, seeds, and nuts. The proteins that it helps synthesize are important for muscle growth and repair. Tryptophan is also converted into serotonin, which is responsible for mood, appetite, and pain reception. It is further down converted into melatonin. Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy. It’s chemical formula is C11H12N2O2. You can find detailed UV spectra of Tryptophan and information about its various lambda maxima by visiting the following link.
Tryptamine is an indolamine derived from tryptophan with the chemical formula C10H12N2. It is naturally found in plants, fungi, and animals. It helps with regulating the activity of dopaminergic, serotonergic, and glutamatergic systems. You can find detailed UV spectra of Tryptamine and information about its various lambda maxima by visiting the following link.
Serotonin is a neurotransmitter derived from tryptophan with the chemical formula C₁₀H₁₂N₂O. Serotonin is a chemical messenger that is crucial for bodily functions. It helps regulate mood and reduce anxiety, digestion, blood clotting, and contributes to the sleep-wake cycle. Low serotonin levels are often associated with a variety of mental illnesses including but not limited to Obsessive Compulsive Disorder (OCD), Post-Traumatic Stress Disorder (PTSD), Depression, and Anxiety.
Dimethyltryptamine is a psychedelic substance derived from tryptamine with the chemical formula C12H16N2. It works as an agonist for Serotonin. It is not approved for medical use. Due to it’s high risk of abuse, it is a Schedule I drug in the United States.
5-MeO-DMT, also known as 5-methoxy-N,N-dimethyltryptamine), O-methylbufotenin, and mebufotenin, is a psychedelic substances derived from tryptamine found in plants and toad secretions. Between 1970s to 1990s, a certain religious sect believed the use of mebufotenin to be sacred to their religious practice. It is currently considered a Schedule I controlled substance in the United States, however.
All the compounds have similar structures and can present difficulties to separation in reverse-phase HPLC. They can be separated using the Primesep 100 mixed-mode column in gradient analysis with acetonitrile (ACN) and water mobile phase with ammonium formate (AmFm) buffer, making the method MS-compatible. The amines can also be UV detected at 280nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 40-50%, 15 min |
| Buffer | Gradient AmFm pH 3.0- 20-80 mM, 15 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm, MS-compatible mobile phase |
| Class of Compounds |
Drug, Hydrophilic, Supplements, Monoamine, Neurotransmitter |
| Analyzing Compounds | Tryptophan, Serotonin, N,N-Dimethyltryptamine, Tryptamine, N,N-Dimethyl-5-methoxytryptamine, N,N-dimethyltryptophan |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
N,N-Dimethyltryptamine
N,N-dimethyltryptophan
Serotonin
Tryptamine
Tryptophan

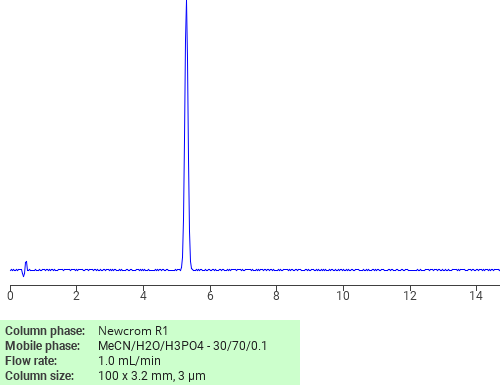
Separation of Tryptamine on Newcrom R1 HPLC column
February 16, 2018
Tryptamine can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options