| CAS Number | 343-65-7 |
|---|---|
| Molecular Formula | C10H12N2O3 |
| Molecular Weight | 208.217 |
| InChI Key | YGPSJZOEDVAXAB-UHFFFAOYSA-N |
| LogP | -2.2 |
| Synonyms |
|
Applications:
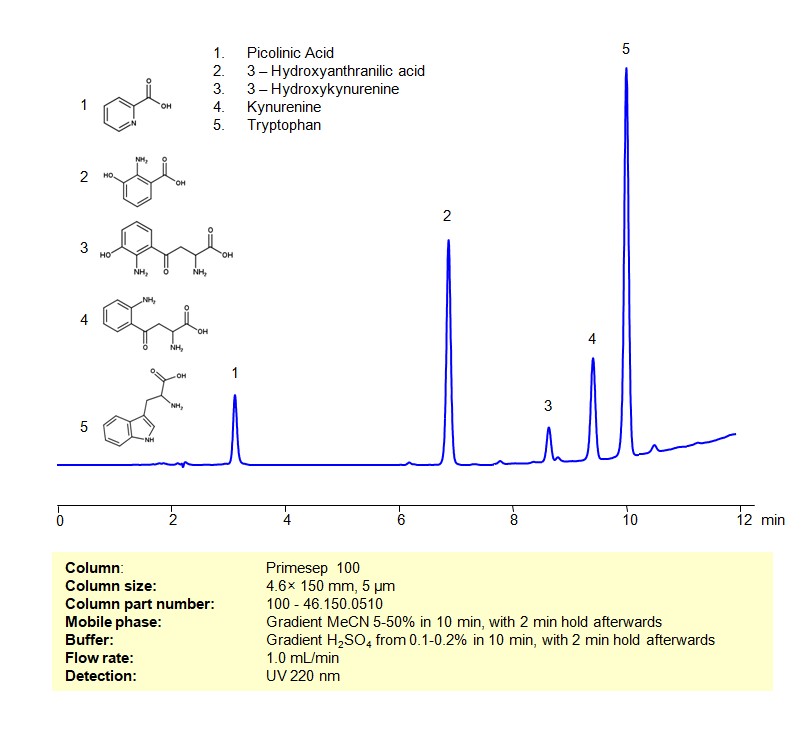
HPLC Method for Analysis of Kynurenine on Primesep 100 Column
August 8, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Kynurenine on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Kynurenine
Kynurenine is a key metabolite in the tryptophan catabolic pathway, often referred to as the kynurenine pathway. This pathway is responsible for breaking down the essential amino acid tryptophan into several bioactive compounds.
Kynurenine can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm.
You can find detailed UV spectra of Kynurenine and information about its various lambda maxima by visiting the following link.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 35% |
| Buffer | H2SO4 -0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Samples | 0.5 mg/ml in H2O – 100% |
| Injection volume | 1 µl |
| LOD* | 20 ppb (250 nm) |
| Class of Compounds | Kynurenines |
| Analyzing Compounds | Kynurenine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

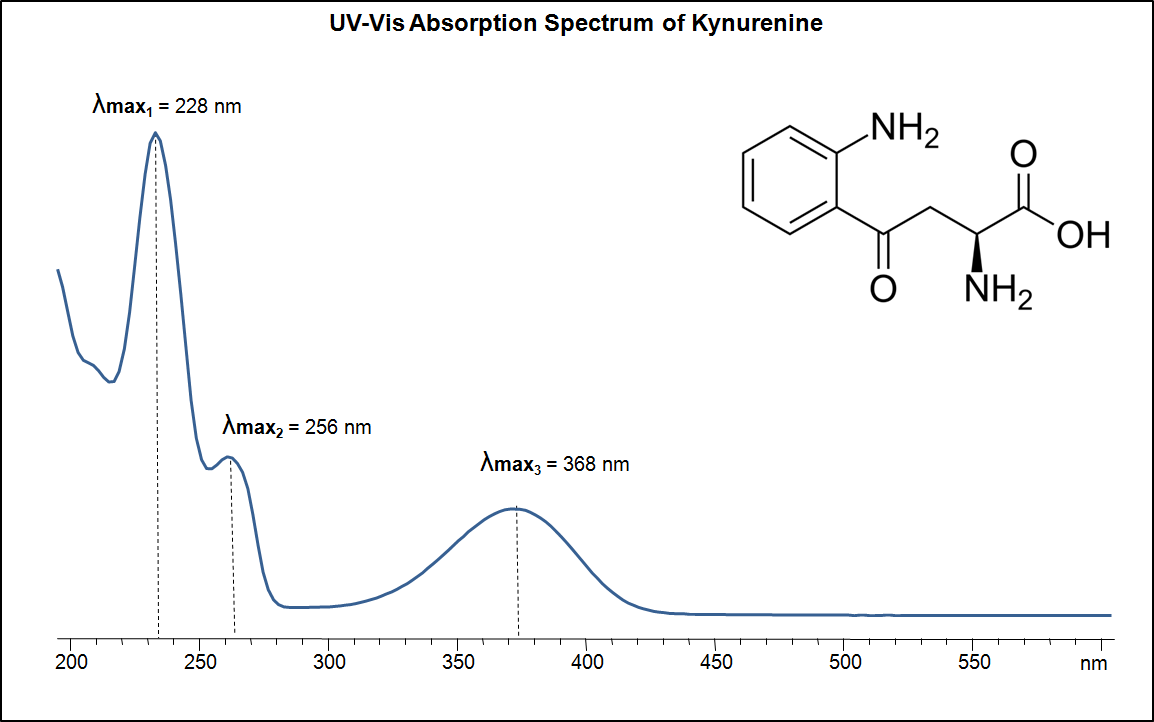
UV-Vis Spectrum of Kynurenine
June 28, 2024
Access the UV-Vis Spectrum SIELC Library
UV-Vis Spectrum of Kynurenine. Absorption Maxima: 228 nm, 256 nm, 368 nm.
If you are looking for optimized HPLC method to analyze Kynurenine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

HPLC Method for Separation of a Mixture of Tryptophan and its Catabolites on Primesep 100 Column
October 3, 2023
High Performance Liquid Chromatography (HPLC) Method for Analysis of Mixture of Tryptophan and its Catabolites on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Tryptophan and its catabolites participate in several biological pathways, having roles in protein synthesis, serving as precursors to bioactive molecules, and influencing several physiological processes. Here’s an overview considering a mixture of tryptophan and its catabolites:
Tryptophan:
- Essential Amino Acid: Tryptophan is a precursor to several important compounds, including serotonin and melatonin.
- In Protein Synthesis: Incorporated into proteins during protein synthesis.
Catabolites:
1. Serotonin:
- Neurotransmitter: Regulates mood, appetite, and sleep, among other functions.
- Derivative: Melatonin, which regulates the sleep-wake cycle.
2. Kynurenine Pathway (Major catabolic pathway of tryptophan):
- Kynurenine: An intermediate and precursor to several bioactive compounds.
- Kynurenic Acid: An NMDA receptor antagonist, believed to have neuroprotective effects.
- Xanthurenic Acid: Its physiological roles are still being explored, but it’s often studied for its relation to diabetes and neurological conditions.
- 3-Hydroxykynurenine: Can generate reactive oxygen species, potentially contributing to cellular stress.
- Quinolinic Acid: A neuroactive metabolite that can act as an NMDA receptor agonist.
3. Indoleamine 2,3-dioxygenase (IDO) Pathway:
- Tryptophan can be degraded into several catabolites via the IDO pathway, influencing immune response and cell proliferation.
.
Tryptophan and its Catabolites can be retained, separated and analyzed on a Primesep 100 mixed-mode stationary phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected using UV at 220 nm.
High Performance Liquid Chromatography (HPLC) Method for Analyses of Mixture of Tryptophan and its Catabolites
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN 5-50% in 10 min, with 2 min hold afterwards |
| Buffer | Gradient H2SO4 from 0.1-0.2% in 10 min, with 2 min hold afterwards |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220 nm |
Description
| Class of Compounds | Essential Amino Acid Tryptophan and its Catabolites |
| Analyzing Compounds | Tryptophan, Picolinic Acid, Kynurenine, 3-Hydroxykynurenine, 3-Hydroxyanthranilic acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
3-Hydroxykynurenine
Kynurenine
Picolinic Acid
Tryptophan

HPLC Analysis of Kynurenine
July 31, 2015
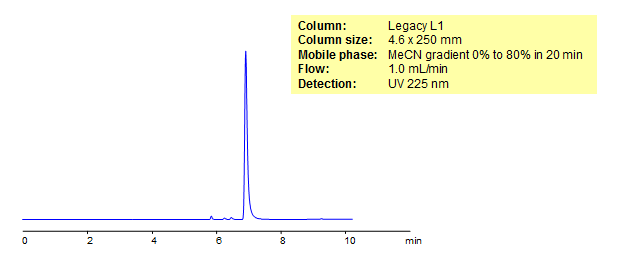
Kynurenine is an important biological compound which carries out a number of biological functions including dilating blood vessles and regulating immune response. Kynurenine was retained on Legacy L1 which containes embedded C18 groups. Comparisons between this chromatagram and from Phenomenex columns are available upon request.
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 0-80%, 20 min |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 225 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Kynurenine |
&
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options