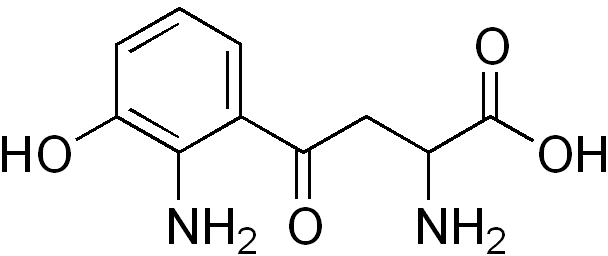
| CAS Number | 484-78-64 |
|---|---|
| Molecular Formula | C10H12N2O4 |
| Molecular Weight | 224.21 |
| InChI Key | VCKPUUFAIGNJHC-UHFFFAOYSA-N |
| LogP | -2.5 |
| Synonyms |
|
Applications:
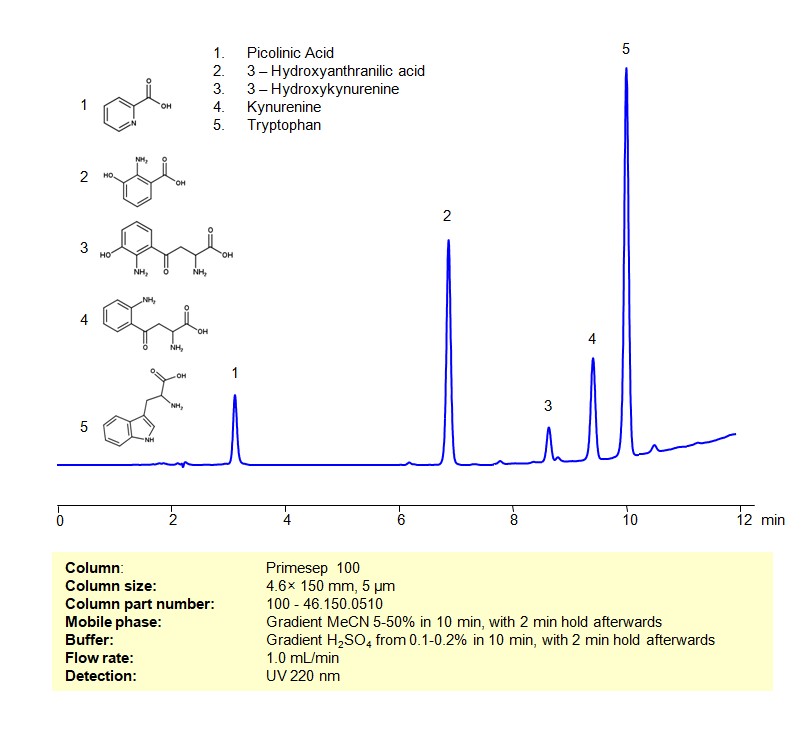
HPLC Method for Separation of a Mixture of Tryptophan and its Catabolites on Primesep 100 Column
October 3, 2023
High Performance Liquid Chromatography (HPLC) Method for Analysis of Mixture of Tryptophan and its Catabolites on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Tryptophan and its catabolites participate in several biological pathways, having roles in protein synthesis, serving as precursors to bioactive molecules, and influencing several physiological processes. Here’s an overview considering a mixture of tryptophan and its catabolites:
Tryptophan:
- Essential Amino Acid: Tryptophan is a precursor to several important compounds, including serotonin and melatonin.
- In Protein Synthesis: Incorporated into proteins during protein synthesis.
Catabolites:
1. Serotonin:
- Neurotransmitter: Regulates mood, appetite, and sleep, among other functions.
- Derivative: Melatonin, which regulates the sleep-wake cycle.
2. Kynurenine Pathway (Major catabolic pathway of tryptophan):
- Kynurenine: An intermediate and precursor to several bioactive compounds.
- Kynurenic Acid: An NMDA receptor antagonist, believed to have neuroprotective effects.
- Xanthurenic Acid: Its physiological roles are still being explored, but it’s often studied for its relation to diabetes and neurological conditions.
- 3-Hydroxykynurenine: Can generate reactive oxygen species, potentially contributing to cellular stress.
- Quinolinic Acid: A neuroactive metabolite that can act as an NMDA receptor agonist.
3. Indoleamine 2,3-dioxygenase (IDO) Pathway:
- Tryptophan can be degraded into several catabolites via the IDO pathway, influencing immune response and cell proliferation.
.
Tryptophan and its Catabolites can be retained, separated and analyzed on a Primesep 100 mixed-mode stationary phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected using UV at 220 nm.
High Performance Liquid Chromatography (HPLC) Method for Analyses of Mixture of Tryptophan and its Catabolites
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN 5-50% in 10 min, with 2 min hold afterwards |
| Buffer | Gradient H2SO4 from 0.1-0.2% in 10 min, with 2 min hold afterwards |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220 nm |
Description
| Class of Compounds | Essential Amino Acid Tryptophan and its Catabolites |
| Analyzing Compounds | Tryptophan, Picolinic Acid, Kynurenine, 3-Hydroxykynurenine, 3-Hydroxyanthranilic acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
3-Hydroxykynurenine
Kynurenine
Picolinic Acid
Tryptophan


