| CAS Number | 63-68-3 |
|---|---|
| Molecular Formula | C5H11NO2S |
| Molecular Weight | 149.211 |
| InChI Key | FFEARJCKVFRZRR-BYPYZUCNSA-N |
| LogP | -1.87 |
| Synonyms |
|
Applications:
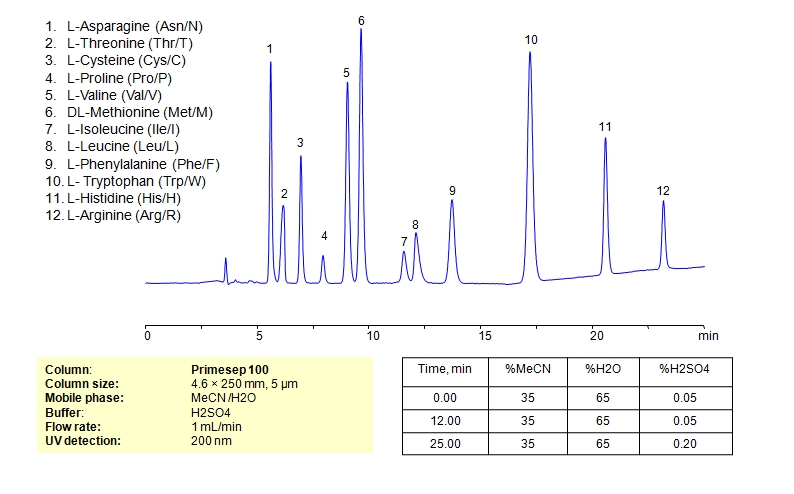
HPLC Separation of Mixture of 12 Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine.
Amino acids are the building blocks of proteins. Based on their dietary requirement, they are classified into essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body in sufficient quantities and must be obtained from the diet. Non-essential amino acids, on the other hand, can be synthesized by the body and are not dependent on dietary intake.
It’s worth noting that while these amino acids are considered “non-essential” for adults under normal circumstances because the body can synthesize them, there are situations where some may become “conditionally essential.” This means that under certain conditions like illness, stress, or trauma, the body might not produce them in sufficient quantities, and dietary intake becomes necessary. Arginine, for instance, is considered conditionally essential, especially during periods of rapid growth, illness, or trauma.
Amino acids can be retained, separeted and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid (H2SO4) as a buffer. This analysis method can be detected in the UV regime at 200 nm.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | H2SO4 0.05% 12 min hold, gradient 0.05-0.20, 13 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Arginine
Asparagine
Cysteine
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Cysteine
L-Methionine
L-Threonine
Leucine
Methionine
Phenylalanine
Proline
Tryptophan
Valine

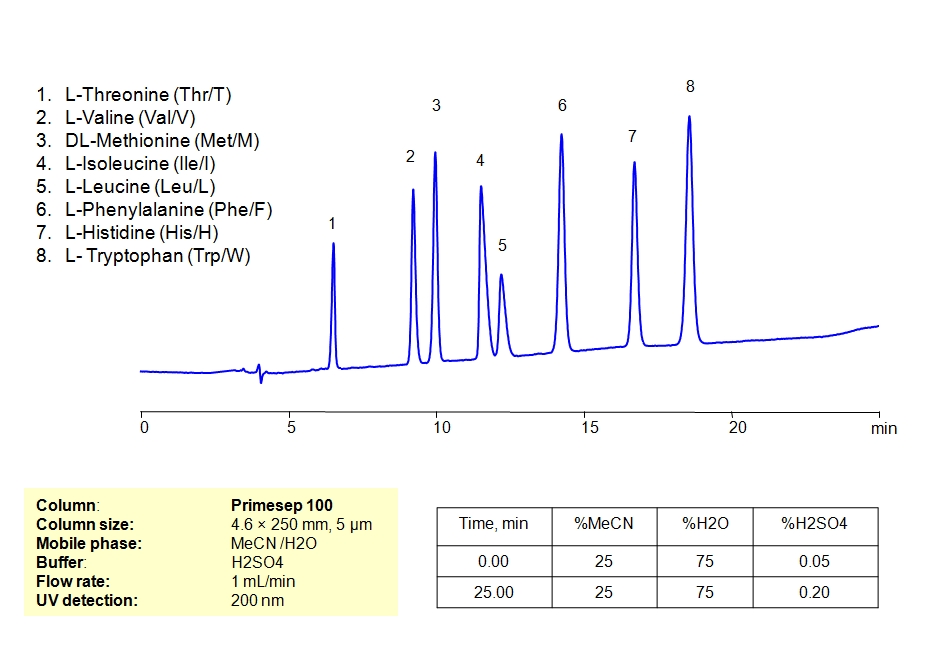
HPLC Separation of Mixture of Essential Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine.
L-Threonine is an essential amino acid with the chemical formula C4H9NO3. It cannot be produced within the body and must be obtained through consuming it. It’s found in many protein-rich foods, including but not limited to eggs, meat, dairy, legumes, and seeds. It is necessary in the body as a building block of protein like collagen and elastin. The two proteins are crucial for skin, hair, and connective issue.
L-Valine is an essential amino acid with the chemical formula C5H11NO2. It cannot be produced within the body and must be obtained through consuming it. It’s found in foods including but not limited to nuts, legumes, whole grains, and seeds. It is especially beneficial for athletes. It is important for muscle repair, growth, and energy regulation.
DL-Methionine is an essential amino acid with the chemical formula C5H11NO2S. It cannot be produced within the body and must be obtained through consuming it. It is required for protein synthesis. It also helps build and repair tissue including, but not limited to, skin, hair, muscles, and nails. In a veterinary context, DL-Methionine is used to address bladder issues in dogs.
L-Isoleucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It is a building block of protein that are essential for muscle growth, repair, and other bodily functions. It also helps regulate blood sugar levels and supports the immune system. It is found in foods like meat, fish, eggs, dairy, beans, lentils, nuts, and seeds.
L-Leucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It stimulates production of protein that are essential for muscle building and repair. Meats are the easiest way to get L-Leucine in significant amounts.
L-Phenylalanine is an essential amino acid with the chemical formula C6H9NO2. It cannot be produced within the body and must be obtained through consuming it. It is typically found in high protein foods such as meat, eggs, and fish. Outside of being important for creation of protein, it is also used in treatment for skin disorders and depression.
L-Histidine is an essential amino acid with the chemical formula C9H11N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts.
L-Tryptophan is an essential amino acid with the chemical formula C11H12N2O2. It cannot be produced within the body and must be obtained through consuming it. Like the other essential proteins, it is a building block for protein and muscle tissue, but it is also converted in the body into serotonin, which affects mood. L-Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy.
Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | Gradient H2SO4 0.05-0.2% 25 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Histidine hydrochloride monohydrate
L-Isoleucine
L-Methionine
L-Threonine
Methionine
Phenylalanine
Tryptophan
Valine

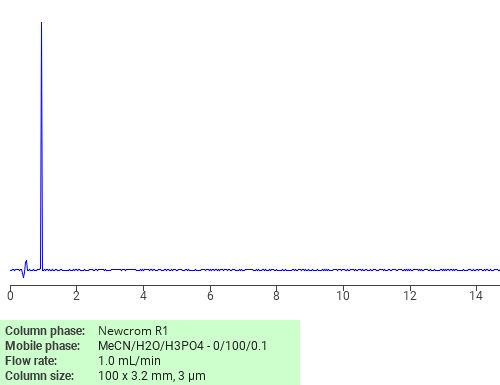
Separation of L-Methionine on Newcrom R1 HPLC column
February 16, 2018
L-Methionine can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options