| CAS Number | 60-00-4 |
|---|---|
| Molecular Formula | C10H16N2O8 |
| Molecular Weight | 292.244 |
| InChI Key | KCXVZYZYPLLWCC-UHFFFAOYSA-N |
| LogP | -1.74 |
| Synonyms |
|
Applications:
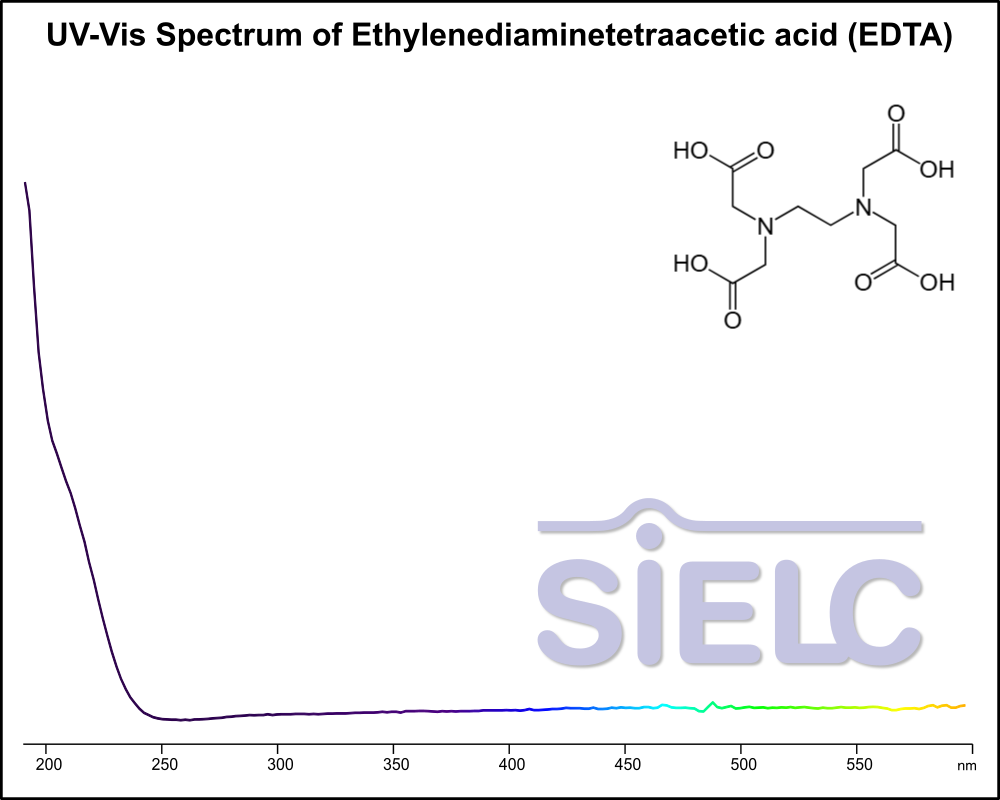
UV-Vis Spectrum of EDTA
December 23, 2025
If you are looking for optimized HPLC method to analyze EDTA (Ethylenediaminetetraacetic Acid) check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

UV-Vis Spectrum of EDTA + Fe Complex
July 12, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze EDTA (Ethylenediaminetetraacetic Acid) check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

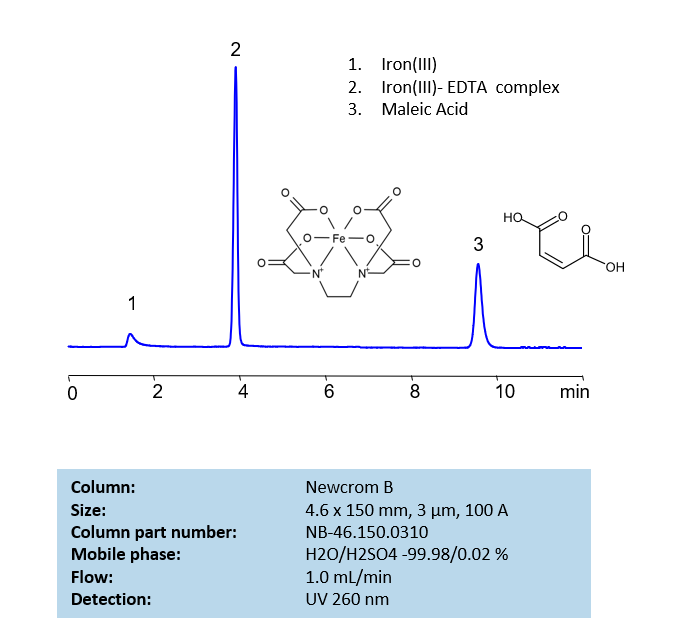
HPLC Determination of EDTA on Newcrom B Column
October 15, 2022
HPLC Method for EDTA (Ethylenediaminetetraacetic Acid) on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA (Ethylenediaminetetraacetic Acid).
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
You can find detailed UV spectra of EDTA (Ethylenediaminetetraacetic Acid) and information about its various lambda maxima by visiting the following link.
EDTA (Ethylenediaminetetraacetic Acid) can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | H2O – 99.98% |
| Buffer | H2SO4 – 0.02% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA (Ethylenediaminetetraacetic Acid) |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method for Analysis of EDTA and Maleic Acid on Newcrom B Column
July 26, 2022
HPLC Method for EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III) on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III).
Ethylenediaminetetraacetic acid (EDTA) is a synthetic amino acid with the chemical formula C10H16N2O8. It is typically used in industry to sequester metal ions, which helps prevent change of colors in textiles and uneven bleaching in paper. Due to it being a chelator, it is also used to soften water during laundry, remove hydrogen sulfide from gas streams, as well as treat mercury and lead poisoning. You can find detailed UV spectra of EDTA + Fe Complex and information about its various lambda maxima by visiting the following link.
Maleic Acid, also known as cis-butenedioic acid, is a dicarboxylic acid with the chemical formula C4H4O4. It is primarily used as a precursor to fumaric acid. It is is derived by hydrolysis of maleic anhydride or is produced by oxidation of benzene or butane. It is occasionally used to stabilize drugs through forming acid addition salts.
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | H2O – 99.98% |
| Buffer | H2SO4 – 0.02% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III) |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Iron(III)
Maleic Acid

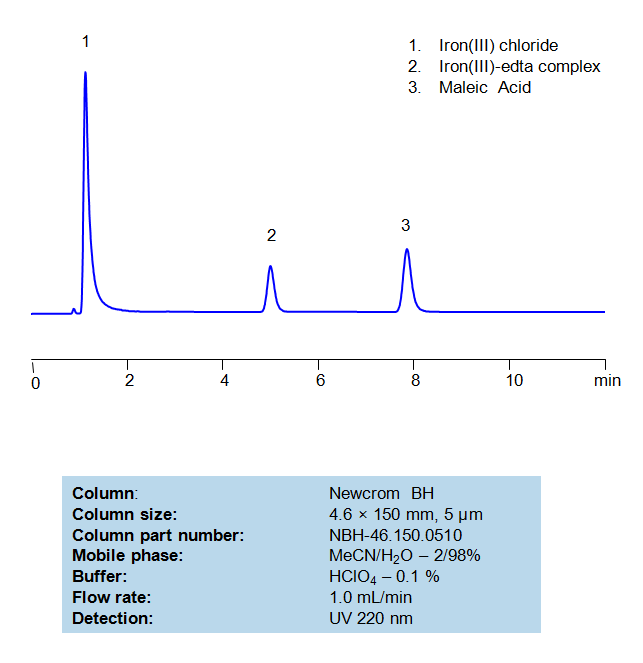
HPLC Method For Analysis Of EDTA and Maleic Acid
April 28, 2022
Separation type: Liquid Chromatography Mixed-mode
Ethylenediaminetetraacetic acid (EDTA) is a very common chelating agent, used particularly for collecting Iron and Calcium ions. It has a wide range of applications, including in the textile industry, paper industry, pharmaceutical industry, and in cosmetics, where it is used to capture unwanted metal ions in solution. Maleic acid, a dicarboxylic acid, has a few interesting applications, such in medicine to form salts with drugs to increase their stability and as a precursor for the production of glyoxylic acid, which is used in cosmetics. An Iron (III)-EDTA complex and Maleic acid can both be retained, separated, and analyzed on a mixed-mode Newcrom BH column with a mobile phase consisting of (mostly) water, Acetonitrile (MeCN), and Perchloric acid (HClO4). This analytical method can be UV detected at 220 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA and Maleic Acid
| Column | Newcrom BH, 4.6×150 mm, 100A |
| Mobile Phase | MeCN – 2% |
| Buffer | HClO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA, Maleic Acid |
Application Column
Newcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsIron(III)
Maleic Acid

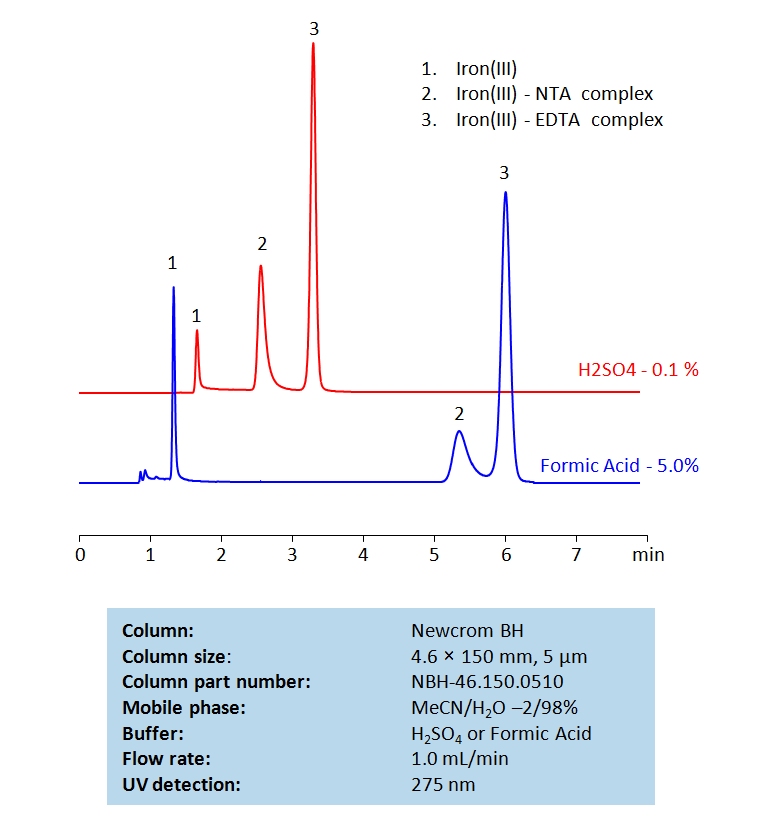
HPLC Determination of Nitrilotriacetic acid (NTA) and Ethylenediaminetetraacetic acid (EDTA) on Newcrom BH Column
September 2, 2021
HPLC Method for Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid).
NTA Standards Solution A: For the preparation of the NTA standard solution, 5 mg of NTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water with sonication. The NTA stock solution (1 mg/mL) should be stored in a cold, dark place and can be used for upto a week to prepare standards of required concentration.
Nitrilotriacetic acid (NTA) and Ethylenediaminetetraacetic acid (EDTA) are two common chelating agents used often in laboratory and medical testing. They are often used to bind to, identify, and analyze metal ions. Below is a method we developed to retain and measure Fe (III) using NTA and EDTA complexes on a Newcrom BH mixed-mode column, with a mobile phase of acetonitrile (MeCN), water, and a buffer of either sulfuric acid (H2SO4) or formic acid:
EDTA Standards Solution B: For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
NTA Standard – Solution A: For the preparation of the NTA standard solution, 5 mg of NTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water with sonication. The NTA stock solution (1 mg/mL) should be stored in a cold, dark place and can be used for upto a week to prepare standards of required concentration.
EDTA Standard – Solution B: For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filter the EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride – Solution C: The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric NTA and EDTA complex analysis: To make a sample for analysis, mix 100 µL each Solution A and Solution B (or unknown sample) with 100 µL Solution C and 700 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
Iron(III) chloride Solution C: The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric NTA and EDTA complexes analysis: To make a sample for analysis mix 100 µL each Solution A and Solution B (or unknown sample) with 100 µL Solution C and 700 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN). Detection is performed using UV.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 2/98% |
| Buffer | H2SO4 or Formic Acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Nitrilotriacetic acid

HPLC Determination of EDTA on Newcrom BH Column
June 17, 2021
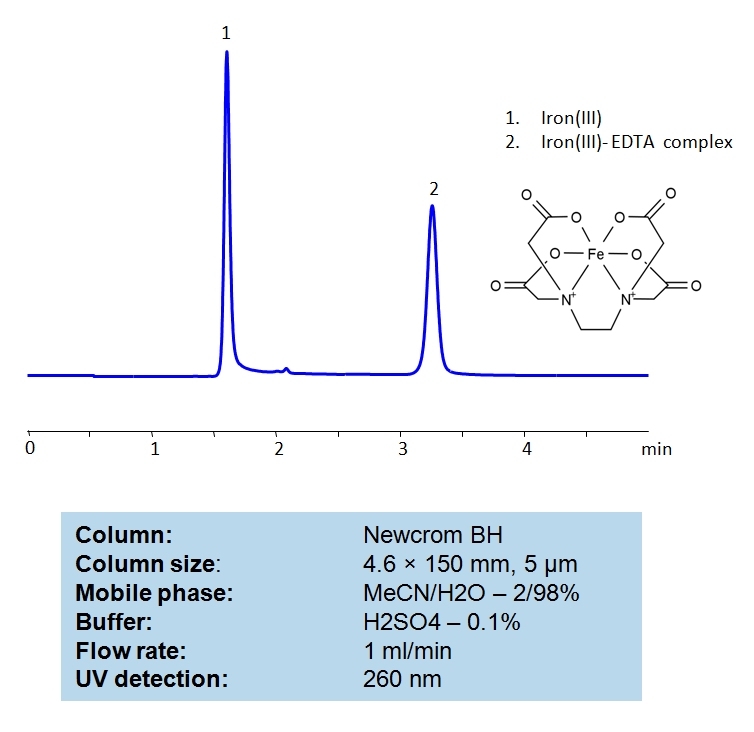
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
| Column | Newcrom BH, 4.6×150 mm, 100A |
| Mobile Phase | MeCN/H2O – 2/98% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA |
Application Column
Newcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsIron(III)

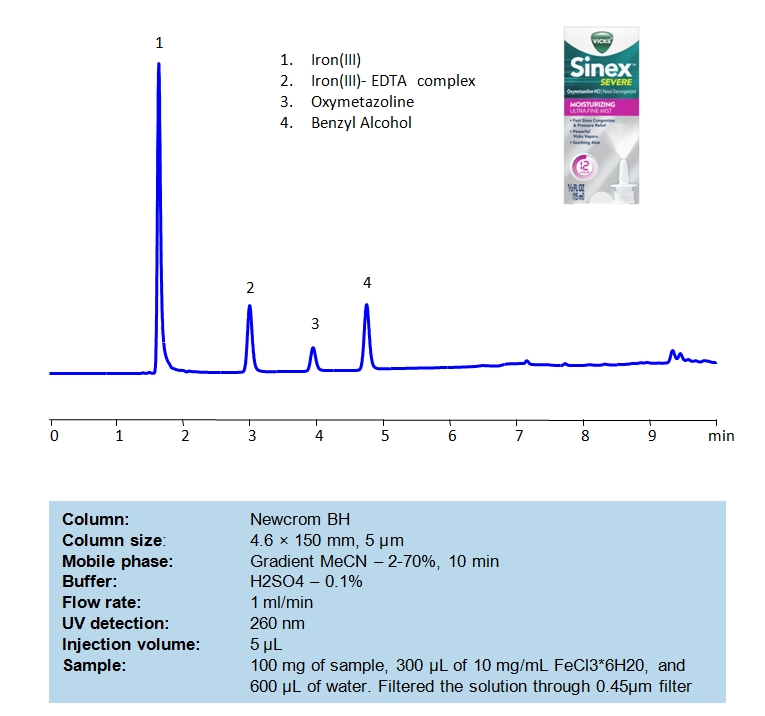
HPLC Separation of EDTA, Oxymetazoline and Benzyl Alcohol in Nasal Spray
September 4, 2020
HPLC Method for EDTA (Ethylenediaminetetraacetic Acid), Oxymetazoline, Benzyl alcohol, Oxymetazoline hydrochloride on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA, Oxymetazoline and Benzyl Alcohol
Benzyl alcohol is found in over-the-counter medications, topical creams, lotions, shampoos, and facial cleansers as an antibacterial, preservative, and/or fungicide. Benzyl alcohol is a colorless liquid with a mild pleasant aromatic scent. Benzyl alcohol is found in many essential oils including jasmine, hyacinth, neroli, rose, and ylang-ylang. Oxymetazoline is a decongestant that shrinks blood vessels in the nasal passages. All active compounds of nasal spray can be separated in HPLC on a Newcrom BH mixed-mode column. The analytical method uses acetonitrile (ACN) gradient and water with sulfuric acid (H2SO4) as buffer and UV detected at 260nm.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 2- 70%, 10 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic, Preservatives |
| Analyzing Compounds | EDTA (Ethylenediaminetetraacetic Acid), Oxymetazoline, Benzyl alcohol, Oxymetazoline hydrochloride |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
EDTA (Ethylenediaminetetraacetic Acid)
Oxymetazoline
Oxymetazoline hydrochloride

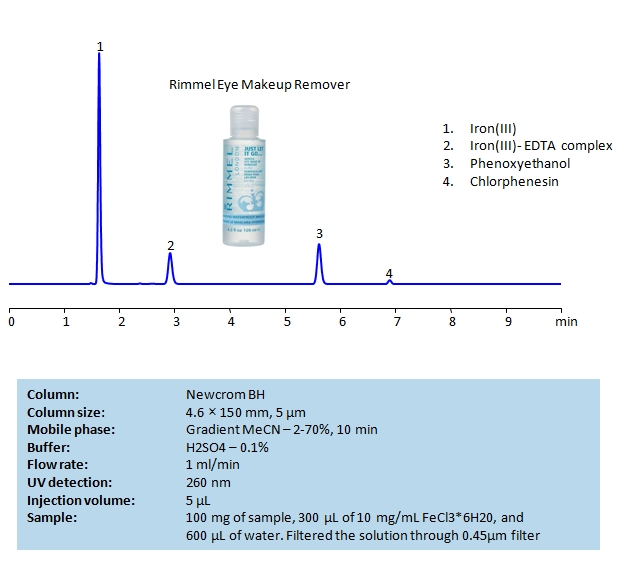
HPLC Determination of EDTA, Phenoxyethanol and Chlorphenesin in Eye Makeup Remover on Newcrom BH Column
August 25, 2020
HPLC Method for Chlorphenesin, 2-Phenoxyethanol, EDTA (Ethylenediaminetetraacetic Acid) on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Chlorphenesin, 2-Phenoxyethanol, EDTA (Ethylenediaminetetraacetic Acid).
Ethylenediaminetetraacetic acid (EDTA) is a synthetic amino acid with the chemical formula C10H16N2O8. It is typically used in industry to sequester metal ions, which helps prevent change of colors in textiles and uneven bleaching in paper. Due to it being a chelator, it is also used to soften water during laundry, remove hydrogen sulfide from gas streams, as well as treat mercury and lead poisoning.
Phenoxyethanol is a synthetic compound with the chemical formula C8H10O2. It is said to have antimicrobial and preservative properties, leading to wide use of it in cosmetics, medicine, and biocides. It is considered safe in the US and Europe at limited concentrations.
Chlorphenesin is a synthetic preservative with the chemical formula C9H11ClO3. It is often used in cosmetics, pharmaceuticals, and hygiene products. It’s preservative properties prevent yeast, fungi, and bacteria from growing in cosmetics as well as lead to it’s occasional use as an anti-fungal treatment. While it is approved for use in United State as the European Union at low concentration, it is banned in Japan. In higher concentrations it can cause nausea, vomiting, and even seizures.
You can find detailed UV spectra of EDTA + Fe complex and information about its various lambda maxima by visiting the following link.
Chlorphenesin, 2-Phenoxyethanol, EDTA (Ethylenediaminetetraacetic Acid) can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes a gradient method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 2- 70%, 10 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic, Preservatives |
| Analyzing Compounds | Chlorphenesin, 2-Phenoxyethanol, EDTA (Ethylenediaminetetraacetic Acid) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Chlorphenesin
EDTA (Ethylenediaminetetraacetic Acid)

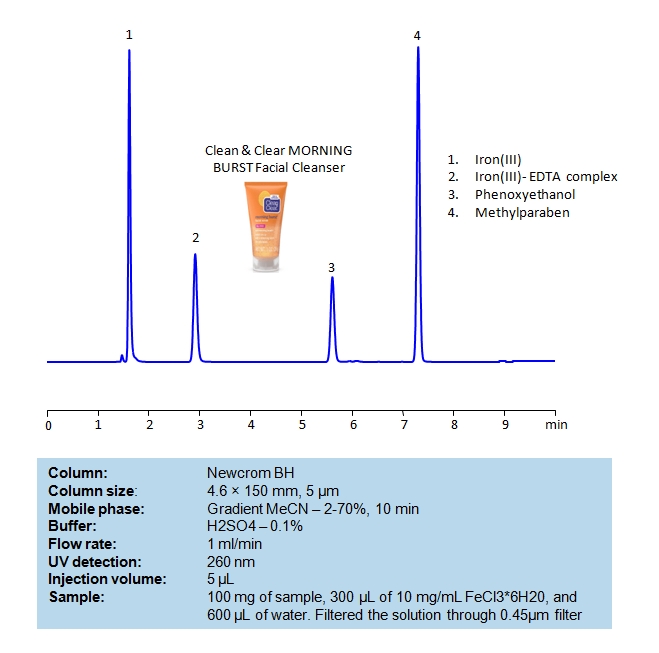
HPLC Determination of EDTA, Phenoxyethanol and Methylparaben in Facial Cleanser on Newcrom BH
August 25, 2020
HPLC Method for EDTA (Ethylenediaminetetraacetic Acid), Methylparaben, Methylparaben sodium, 2-Phenoxyethanol on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA (Ethylenediaminetetraacetic Acid), Methylparaben, Methylparaben sodium, 2-Phenoxyethanol.
Ethylenediaminetetraacetic acid (EDTA) is a synthetic amino acid with the chemical formula C10H16N2O8. It is typically used in industry to sequester metal ions, which helps prevent change of colors in textiles and uneven bleaching in paper. Due to it being a chelator, it is also used to soften water during laundry, remove hydrogen sulfide from gas streams, as well as treat mercury and lead poisoning.
Phenoxyethanol is a synthetic compound with the chemical formula C8H10O2. It is said to have antimicrobial and preservative properties, leading to wide use of it in cosmetics, medicine, and biocides. It is considered safe in the US and Europe at limited concentrations.
Methylparaben, also known as Methyl 4-hydroxybenzoate, is a paraben with the chemical formula C8H8O3. It is used as a preservative in food, cosmetics, and pharmaceuticals as it is said to have antimicrobial and antifungal properties. It is considered safe for use in low concentrations, but it may cause irritation or contact dermatitis in rare cases and for those who are allergic.
You can find detailed UV spectra of EDTA + Fe complex and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Methylparaben and information about its various lambda maxima by visiting the following link.
EDTA (Ethylenediaminetetraacetic Acid), Methylparaben, Methylparaben sodium, 2-Phenoxyethanol can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes a gradient method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 2- 70%, 10 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic, Preservatives |
| Analyzing Compounds | EDTA (Ethylenediaminetetraacetic Acid), Methylparaben, Methylparaben sodium, 2-Phenoxyethanol |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
EDTA (Ethylenediaminetetraacetic Acid)
Methylparaben
Methylparaben sodium

HPLC Determination of EDTA on Newcrom B Column
April 22, 2020
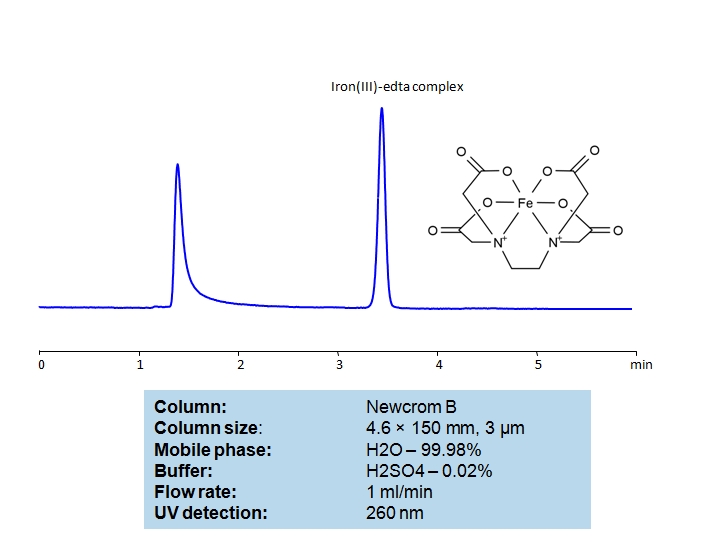
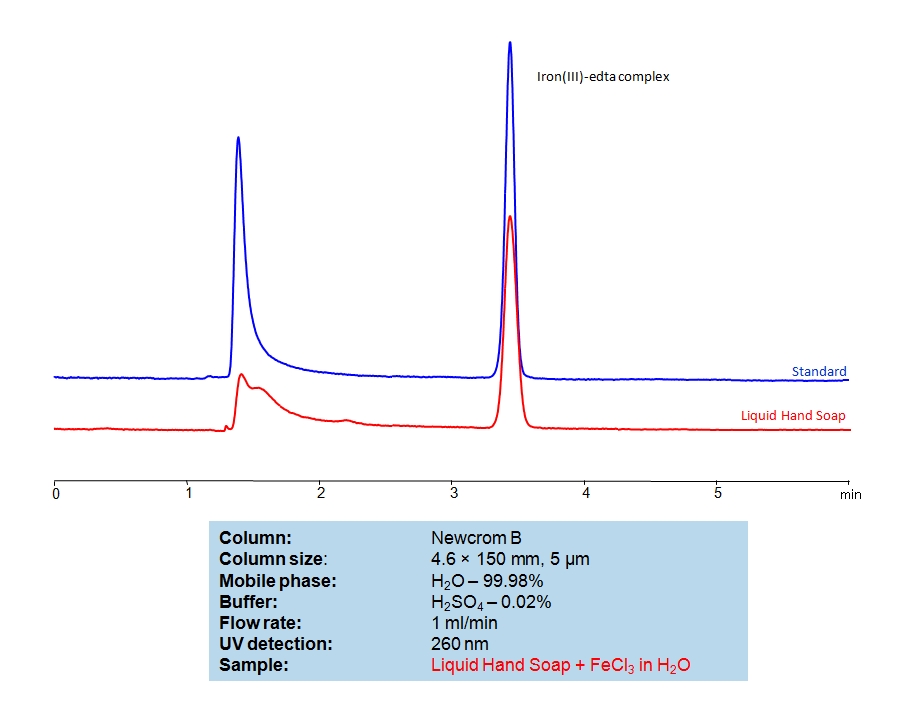
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
Ethylenediaminetetraacetic acid (EDTA) is a very common chelating agent, used particularly for collecting Iron and Calcium ions. It has a wide range of applications, including in the textile industry, paper industry, pharmaceutical industry, and in cosmetics, where it is used to capture unwanted metal ions in solution. An Iron (III)-EDTA complex can be retained and analyzed on a mixed-mode Newcrom B column with a mobile phase consisting of (mostly) water, Acetonitrile (MeCN), and Sulfuric acid (H2SO4). This analytical method can be UV detected at 260 nm with high resolution and peak symmetry.
| Column | Newcrom B, 4.6×150 mm, 100A |
| Mobile Phase | H2O – 99.98% |
| Buffer | H2SO4 – 0.02% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA |
Application Column
Newcrom B
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options
HPLC Retention of EDTA in Sulfated CD
November 21, 2010

EDTA, or Ethylenediaminetetraacetic acid, is widely used in textile, pulp/paper, food and pharmaceutical industries. It has application as chelating agent and preservative. EDTA molecule is very hydrophilic and contains two basic groups and four carboxylic groups. It has tendency to bind to metals, making analysis of EDTA by HPLC very challenging. EDTA is analyzed in the presence of copper sulfate as visualization agent on a Primesep SB column. The method can be used for EDTA determination in various mixtures and compositions using UV detector.
| Column | Primesep SB , 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | MeSO3H with CuSO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 300 nm |
| Class of Compounds |
Acid |
| Analyzing Compounds | EDTA, or Ethylenediaminetetraacetic acid |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
HILIC Separation of Common Preservatives – Citric Acid, Ascorbic Acid and EDTA
July 16, 2009
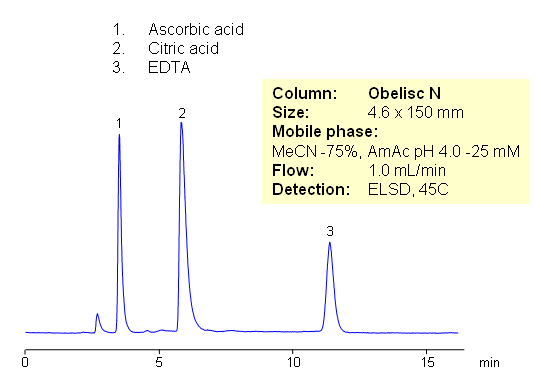
Citric acid, ascorbic acid, and EDTA are commonly used in food and pharmaceutical industry as preservatives. These compounds are very polar in nature. They are weak organic acids with limited UV activity. Retention and separation is achieved on HILIC mixed-mode Obelisc N column. All three compounds are retained by combination of strong HILIC and strong anion-exchange mechanisms. Separation can be monitored by ELSD, LC/MS, UV or Corona CAD. In contrast to other HILIC column, Obelisc N has two ionizable groups basic and acidic which provide ion-exchange interaction in addition to hydrophilic interaction. This allows to use less acetonitrile for HILIC separation.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Citric acid, ascorbic acid and EDTA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsEDTA (Ethylenediaminetetraacetic Acid)
Vitamin C

HPLC Application for Analysis of EDTA
December 6, 2007
New more reliable method for EDTA analysis is available
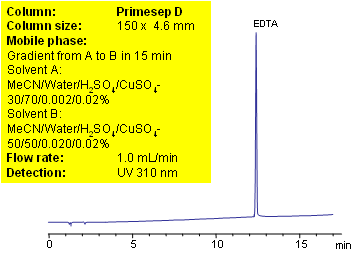
EDTA (Ethylenediaminetetraacetic acid) is amino acid based compound which is widely used in industrial cleaners, detergents, photography, agrochemicals, textile, and food industries. EDTA serves as preservative in packaged food, vitamins and pharmaceutical formulations. It is used in organic and analytical laboratories as scavenger of metals, buffer solution, complexometric titration, masking agent for metal determination, etc.
EDTA is very polar compound with strong chelating properties. Low UV activity and strong binding to metal ions makes HPLC analysis of EDTA very difficult. Reproducible method for determination of wide range on concentration of EDTA in various formulations is developed using Primesep D anion-exchange mixed mode column. Copper sulfate is used for visualization purposes to increase sensitivity of the method. EDTA forms UV active complex with some metal ions. This method can be used for highly accurate quantitation of EDTA in different mixtures and composition. Method demonstrates controllable retention and perfect peak shape. Equilibration of the column prior to analysis requires special attention.
If multiple injections produce different retention times, your column might not be equilibrated properly. Primesep D columns have a large ionic capacity towards anions. The shipping solvent for the Primesep D columns is ACN/water/TFA. The end point of gradient has only 4 mmol of sulfate ions. It takes over 5 hours to equilibrate a column with such low concentration. Before running your experiments you need to wash your column with 20% ACN and 0.2% of sulfuric acid for 1 hour. After you replace previous buffer/additive, you can set up your experiment. If you don’t change ionic modifier in your mobile phase the equilibration time is equivalent of 4-5 column volumes.
| Column | Primesep D , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 with CuSO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 310 nm |
| Class of Compounds |
Acid |
| Analyzing Compounds | EDTA, or Ethylenediaminetetraacetic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options