HPLC Method for Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid).
NTA Standards Solution A: For the preparation of the NTA standard solution, 5 mg of NTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water with sonication. The NTA stock solution (1 mg/mL) should be stored in a cold, dark place and can be used for upto a week to prepare standards of required concentration.
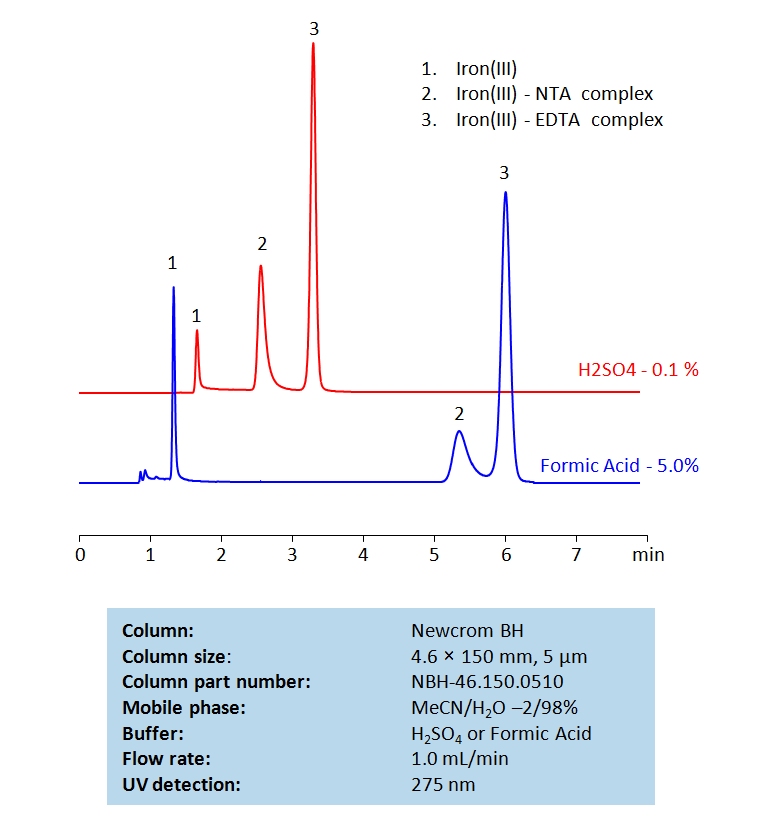
Nitrilotriacetic acid (NTA) and Ethylenediaminetetraacetic acid (EDTA) are two common chelating agents used often in laboratory and medical testing. They are often used to bind to, identify, and analyze metal ions. Below is a method we developed to retain and measure Fe (III) using NTA and EDTA complexes on a Newcrom BH mixed-mode column, with a mobile phase of acetonitrile (MeCN), water, and a buffer of either sulfuric acid (H2SO4) or formic acid:
EDTA Standards Solution B: For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
NTA Standard – Solution A: For the preparation of the NTA standard solution, 5 mg of NTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water with sonication. The NTA stock solution (1 mg/mL) should be stored in a cold, dark place and can be used for upto a week to prepare standards of required concentration.
EDTA Standard – Solution B: For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filter the EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride – Solution C: The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric NTA and EDTA complex analysis: To make a sample for analysis, mix 100 µL each Solution A and Solution B (or unknown sample) with 100 µL Solution C and 700 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
Iron(III) chloride Solution C: The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric NTA and EDTA complexes analysis: To make a sample for analysis mix 100 µL each Solution A and Solution B (or unknown sample) with 100 µL Solution C and 700 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN). Detection is performed using UV.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 2/98% |
| Buffer | H2SO4 or Formic Acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | Nitrilotriacetic acid, EDTA (Ethylenediaminetetraacetic Acid) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Nitrilotriacetic acid