Applications:
HPLC Determination of EDTA on Newcrom BH Column
October 30, 2025
HPLC Method for Iron(III) on Newcrom BH by SIELC Technologies
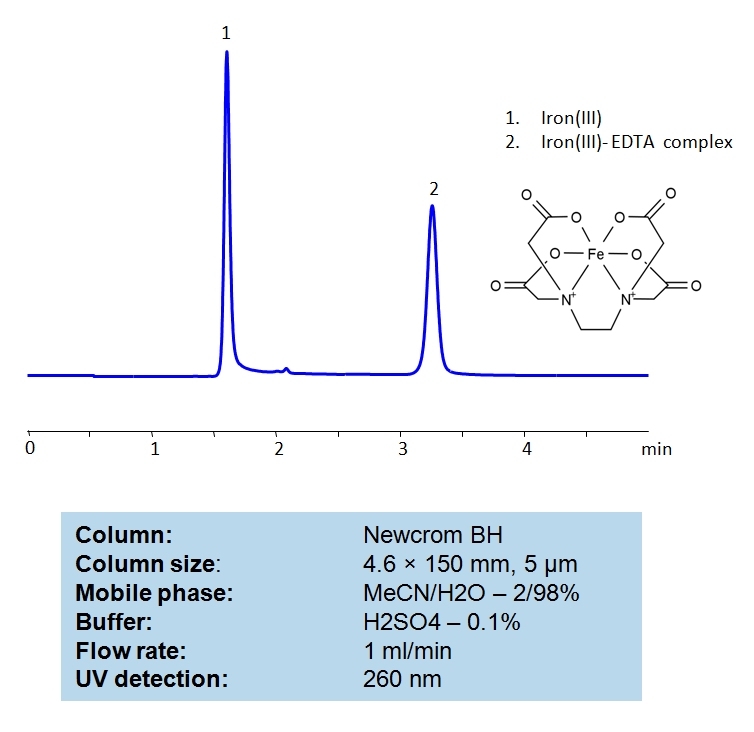
High Performance Liquid Chromatography (HPLC) Method for Analysis of Iron(III).
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 2/98% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | Iron(III) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

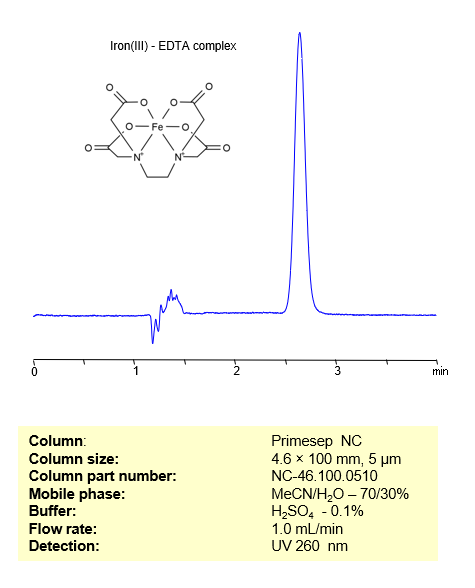
HPLC Method for Analysis of Iron (III) in FeCl3 on Primesep NC Column
June 23, 2023
HPLC Method for Analysis of Iron(III) on Primesep NC by SIELC Technologies
Separation type: Hydrophilic interaction liquid chromatography (HILIC)
The analysis of Fe3+ involves various analytical methods such as colorimetric methods, atomic absorption spectroscopy (AAS), inductively coupled plasma optical emission spectrometry (ICP-OES), and high-performance liquid chromatography (HPLC). Within the realm of HPLC, specific techniques like ion chromatography or chelation chromatography are often employed to separate Fe3+ ions. Post-separation, UV-visible detectors or post-column derivatization with appropriate reagents can be used for detection and quantification of Fe3+ ions.
To overcome these complexities, SIELC has developed a novel, selective methodology, offering a straightforward and reliable approach for Fe(III) quantitation in various liquid samples. This method revolves around the formation of a complex with EDTA, which exhibits strong UV light absorption with peak maxima at 260 nm
EDTA Standards Solution A
For the preparation of the Disodium Dihydrogen Ethylenediaminetetraacetate Dihydrate (EDTA disodium salt) standard solution, 20 mg of EDTA was accurately weighed and transferred into a 10 mL volumetric flask and dissolved in water with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (2000 ppm) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B
The standard stock solution of Iron(III) chloride (100 ppm Fe3+) was prepared in water. 48 mg of Iron(III) chloride hexahydrate was accurately weighed and transferred into a 100 mL volumetric flask and dissolved in water and sonicated if needed.
General procedure for Iron(III) – EDTA complex analysis
Mix 100 µL Solution A, 200 µL Solution B, and 700 µL of water. Place this mixture in a plastic HPLC vial for analysis.
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Iron(III)
Condition
| Column | Primesep NC, 4.6*100, 5 µm |
| Mobile Phase | MeCN/H2O -70/30% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time | 2.8 min |
| Sample concentration | 100 ppm |
| Injection volume | 10 µl |
| Sample diluent | H2O + NaOH |
| LOD | 1 ppm |
Description
| Class of Compounds | Ion, Metal |
| Analyzing Compounds | Iron(III) |

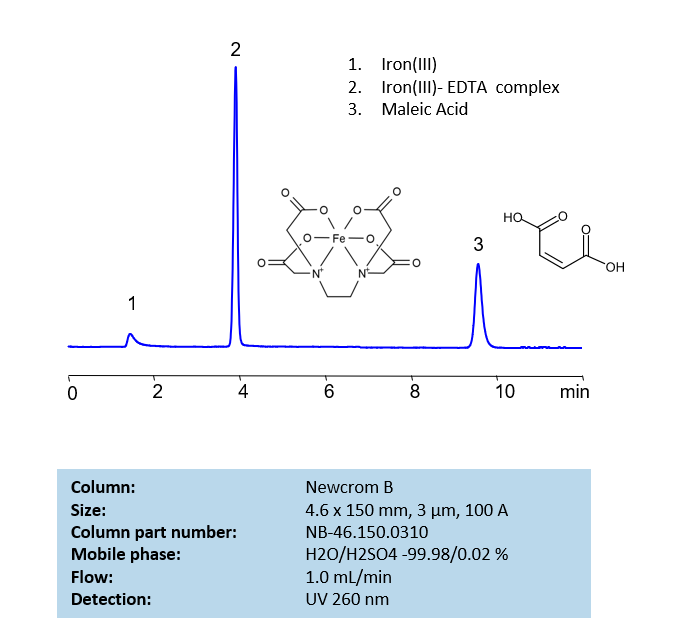
HPLC Method for Analysis of EDTA and Maleic Acid on Newcrom B Column
July 26, 2022
HPLC Method for EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III) on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III).
Ethylenediaminetetraacetic acid (EDTA) is a synthetic amino acid with the chemical formula C10H16N2O8. It is typically used in industry to sequester metal ions, which helps prevent change of colors in textiles and uneven bleaching in paper. Due to it being a chelator, it is also used to soften water during laundry, remove hydrogen sulfide from gas streams, as well as treat mercury and lead poisoning. You can find detailed UV spectra of EDTA + Fe Complex and information about its various lambda maxima by visiting the following link.
Maleic Acid, also known as cis-butenedioic acid, is a dicarboxylic acid with the chemical formula C4H4O4. It is primarily used as a precursor to fumaric acid. It is is derived by hydrolysis of maleic anhydride or is produced by oxidation of benzene or butane. It is occasionally used to stabilize drugs through forming acid addition salts.
EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | H2O – 99.98% |
| Buffer | H2SO4 – 0.02% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA (Ethylenediaminetetraacetic Acid), Maleic Acid, Iron(III) |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Iron(III)
Maleic Acid

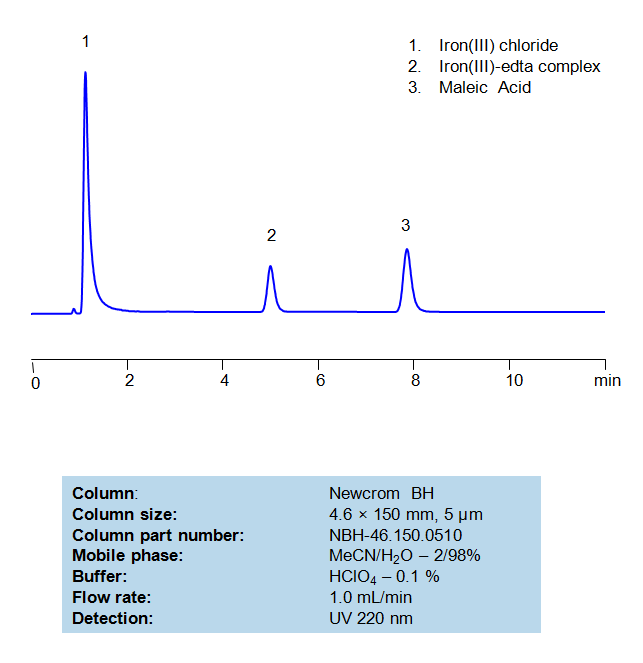
HPLC Method For Analysis Of EDTA and Maleic Acid
April 28, 2022
Separation type: Liquid Chromatography Mixed-mode
Ethylenediaminetetraacetic acid (EDTA) is a very common chelating agent, used particularly for collecting Iron and Calcium ions. It has a wide range of applications, including in the textile industry, paper industry, pharmaceutical industry, and in cosmetics, where it is used to capture unwanted metal ions in solution. Maleic acid, a dicarboxylic acid, has a few interesting applications, such in medicine to form salts with drugs to increase their stability and as a precursor for the production of glyoxylic acid, which is used in cosmetics. An Iron (III)-EDTA complex and Maleic acid can both be retained, separated, and analyzed on a mixed-mode Newcrom BH column with a mobile phase consisting of (mostly) water, Acetonitrile (MeCN), and Perchloric acid (HClO4). This analytical method can be UV detected at 220 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of EDTA and Maleic Acid
| Column | Newcrom BH, 4.6×150 mm, 100A |
| Mobile Phase | MeCN – 2% |
| Buffer | HClO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA, Maleic Acid |
Application Column
Newcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsIron(III)
Maleic Acid

HPLC Determination of EDTA on Newcrom BH Column
June 17, 2021

EDTA Standards Solution A:
For the preparation of the EDTA standard solution, 5 mg of EDTA was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in 0.001N NaOH water solution with sonication or magnetic stirrer mixing. Filtered The EDTA stock solution (1.0 mg/mL) should be stored in a cold dark place and can be used for a week to prepare standards of required concentration.
Iron(III) chloride Solution B:
The standard stock solution of Iron(III) chloride (10 mg/ml) was prepared in water. 50 mg of FeCl3 was accurately weighed and transferred into a 5 mL volumetric flask and dissolved in water, with sonication if needed.
General procedure for Ferric EDTA complex analysis:
To make a sample for analysis mix 100 µL Solution A (or unknown sample) with 100 µL Solution B and 800 µL of water. Place this mixture in a plastic HPLC vial for analysis. Setup instrument and column according to the method provided.
| Column | Newcrom BH, 4.6×150 mm, 100A |
| Mobile Phase | MeCN/H2O – 2/98% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | EDTA |
Application Column
Newcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsIron(III)