| CAS Number | 59-30-3 |
|---|---|
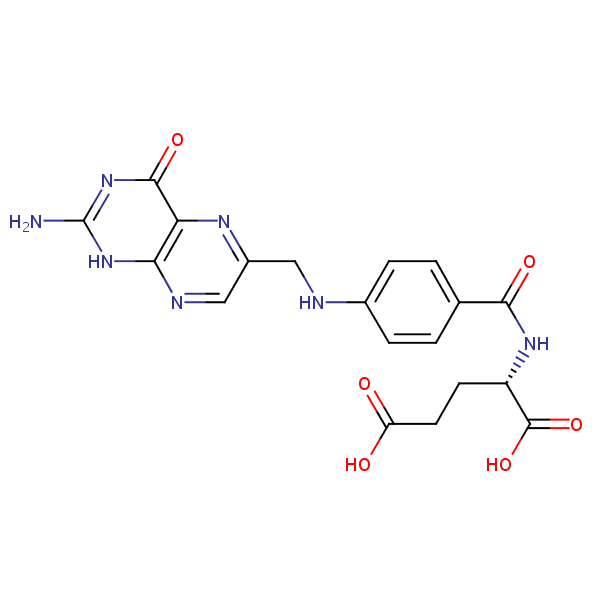
| Molecular Formula | C19H19N7O6 |
| Molecular Weight | 441.404 |
| InChI Key | OVBPIULPVIDEAO-LBPRGKRZSA-N |
| LogP | -1.77 |
| Synonyms |
|
Applications:
HPLC Method for Separation of 10 Water-Soluble Vitamins on Chromni Column
September 17, 2025
HPLC Method for Separation of 10 Water-Soluble Vitamins on Chromni by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for separation of Vitamin B2 (Riboflavin), Nicotinic Acid/Niacin (3-pyridinecarboxylic acid), Nicotinamide, Vitamin B6 (Pyridoxine), Folic Acid, Cyanocobalamin, Thiamine diphosphate (Thiamine pyrophosphate), Vitamin B1 (Thiamine), Pantothenic Acid (Vitamin B5), Biotin
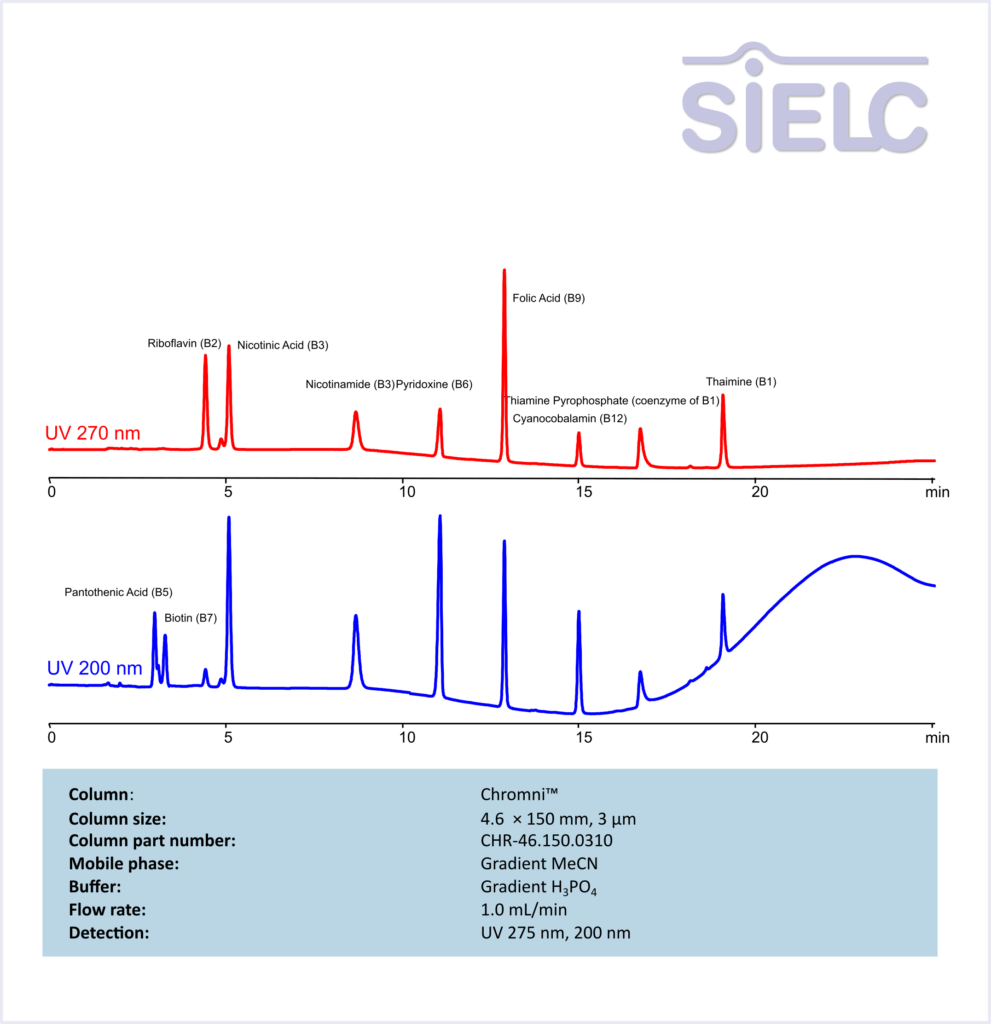
Riboflavin (B2), Nicotinic Acid (B3), Nicotinamide (B3), Pyridoxine (B6), Folic Acid (B9), Cyanocobalamin (B12), Thiamine Pyrophosphate (coenzyme of B1), Thiamine (B1), Pantothenic Acid (B5), Biotin (B7) are water soluble vitamins with a key function of energy metabolism. These coenzymes are responsible for converting food into usable energy.
Vitamin B2 (Riboflavin), Nicotinic Acid/Niacin (3-pyridinecarboxylic acid), Nicotinamide, Vitamin B6 (Pyridoxine), Folic Acid, Cyanocobalamin, Thiamine diphosphate (Thiamine pyrophosphate), Vitamin B1 (Thiamine), Pantothenic Acid (Vitamin B5), Biotin can be retained and analyzed using the Chromni stationary phase column. The analysis utilizes a gradient method with a simple mobile phase consisting of water, acetonitrile (MeCN). Detection is performed using UV.
| Column | Chromni, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – see table |
| Buffer | H3PO4 – see table |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275 nm, 200 nm |
Gradient Elution Program for HPLC Method
| Time (min) | A – H2O (%) | B – MeCN (%) | C – H3PO4 1% in H2O (%) | Notes |
| 0 | 0 | 90 | 10 | Starting Conditions (Hold) |
| 4 | 0 | 90 | 10 | Gradient Start |
| 20 | 20 | 30 | 50 | Gradient End |
| 20.01 | 0 | 90 | 10 | Column Equilibration |
| 30 | 0 | 90 | 10 | End of Run |
| Class of Compounds | Vitamins |
| Analyzing Compounds | Vitamin B2 (Riboflavin), Nicotinic Acid/Niacin (3-pyridinecarboxylic acid), Nicotinamide, Vitamin B6 (Pyridoxine), Folic Acid, Cyanocobalamin, Thiamine diphosphate (Thiamine pyrophosphate), Vitamin B1 (Thiamine), Pantothenic Acid (Vitamin B5), Biotin |
Application Column
Chromni
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Cyanocobalamin
Folic Acid
Nicotinamide
Nicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Pantothenic Acid (Vitamin B5)
Thiamine diphosphate (Thiamine pyrophosphate)
Vitamin B1 (Thiamine)
Vitamin B2 (Riboflavin)
Vitamin B6 (Pyridoxine)

UV-Vis Spectrum of Folic Acid
July 12, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Folic Acid check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

HPLC Method for Separation of Folic Acid, Methotrexate and Aminopterin on Primesep 100 Column
June 12, 2023
HPLC Method for Separation of Methotrexate, Aminopterin, Folic Acid on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Aminopterin and folic acid are chemically related compounds that play significant roles in biochemistry and medicine.
Methotrexate, Aminopterin, and Folic Acid are chemically related compounds that have important roles in biochemistry and medicine.
- Methotrexate: This is a chemotherapy agent and immune system suppressant. Methotrexate is an antimetabolite and antifolate drug, meaning it inhibits the metabolism of folic acid. It does this by inhibiting the enzyme dihydrofolate reductase (DHFR), preventing the formation of active folate and thus interfering with DNA and RNA synthesis. It is used to treat certain types of cancer and autoimmune diseases like rheumatoid arthritis and psoriasis.
- Aminopterin: This is also an antifolate, and it works in a similar way to methotrexate, by inhibiting the enzyme DHFR. Aminopterin was one of the first antifolates to be used in cancer chemotherapy. However, it has largely been replaced by methotrexate, which is less toxic and more effective.
- Folic Acid: Also known as folate or vitamin B9, this is an essential nutrient that the body needs for many functions. It’s critical for the synthesis, repair, and functioning of DNA, as well as for cell division and growth. A deficiency of folic acid can lead to anemia and other health problems.
The antifolate drugs methotrexate and aminopterin are designed to interfere with the actions of folic acid, making them effective in slowing down the growth of rapidly dividing cells, such as cancer cells. However, this can also affect normal, healthy cells, leading to side effects.
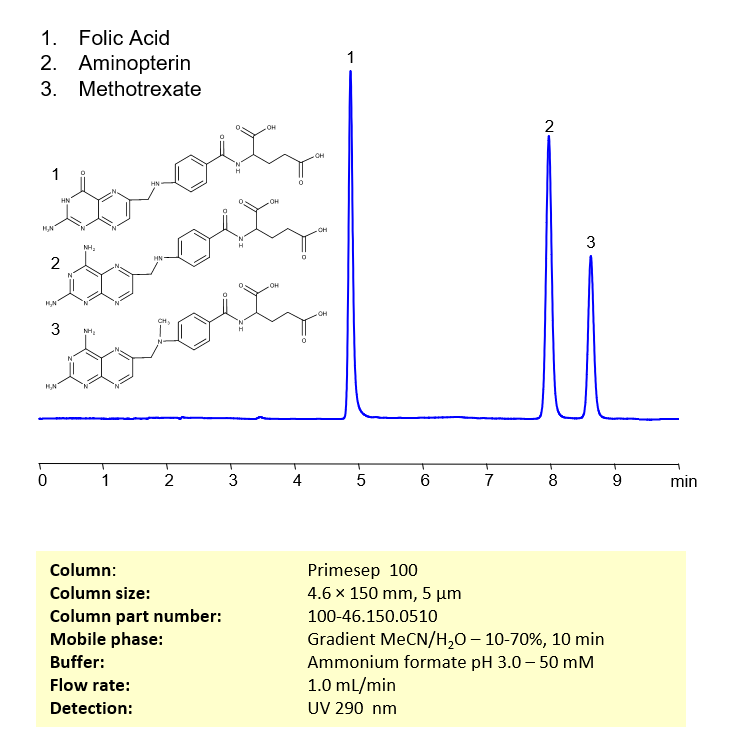
Aminopterin, methotrexate and folic acid can be retained, separated, and analyzed using a reverse-phase Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and ammonium formate, which serves as a buffer. This analytical method can be monitored using UV detection at 290 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Methotrexate, Aminopterin, Folic Acid
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN -10-70%, 10 min |
| Buffer | Ammonium formate pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 290 nm |
| Peak Retention Time | 5.08 min, 8.32 min |
| Sample concentration | 0.02 mg/ml |
| Injection volume | 5 µl |
| Sample diluent | H2O+NaOH |
| LOD | 0.04 ppm |
Description
| Class of Compounds | Drug, Aminopterin, Antifolates, Acid |
| Analyzing Compounds | Methotrexate, Aminopterin, Folic Acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Folic Acid
Methotrexate

HPLC Method for Separation of Biopterin, Rhamnopterin, Neopterin, Folic Acid and Aminopterin on Primesep 100 Column
June 8, 2023
HPLC Method for Analysis of Biopterin, Rhamnopterin, Neopterin, Folic Acid, Aminopterin on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
These compounds, including Biopterin, Rhamnopterin, Neopterin, Folic Acid, and Aminopterin, are all related to the chemical structure known as pterin. Pterins are a group of heterocyclic compounds composed of a pteridine ring system, which consists of two fused six-membered rings, a pyrimidine ring and a pyrazine ring. Here’s a brief overview of each:
- Biopterin (or Tetrahydrobiopterin): This is a coenzyme in the synthesis of several neurotransmitters, including dopamine, norepinephrine, and serotonin. It also plays a role in the production of nitric oxide. Deficiencies in the enzyme that recycles tetrahydrobiopterin can lead to phenylketonuria (PKU), a metabolic disorder.
- Rhamnopterin:
- Neopterin: This pteridine derivative is produced by the human immune system upon activation by interferon-gamma and serves as a marker for immune system activation. Elevated levels can be found in various disorders, such as viral infections, autoimmune diseases, or malignancies.
- Folic Acid: Also known as vitamin B9, folic acid is crucial for many functions in the body, including DNA synthesis and repair, cell division, and growth. Folic acid must be obtained through diet or supplementation, as humans cannot synthesize it.
- Aminopterin: An antifolate (folic acid antagonist), aminopterin was one of the first chemotherapy drugs used to treat cancer. It works by inhibiting dihydrofolate reductase, an enzyme that converts folic acid to its active form, thereby interfering with cell growth and division. Aminopterin is highly toxic and has been largely replaced by less toxic and more effective drugs, such as methotrexate.
- It’s important to note that while these compounds all contain a pteridine ring, they have very different biological activities and uses. Always consult with a healthcare provider or relevant professionals for accurate information.
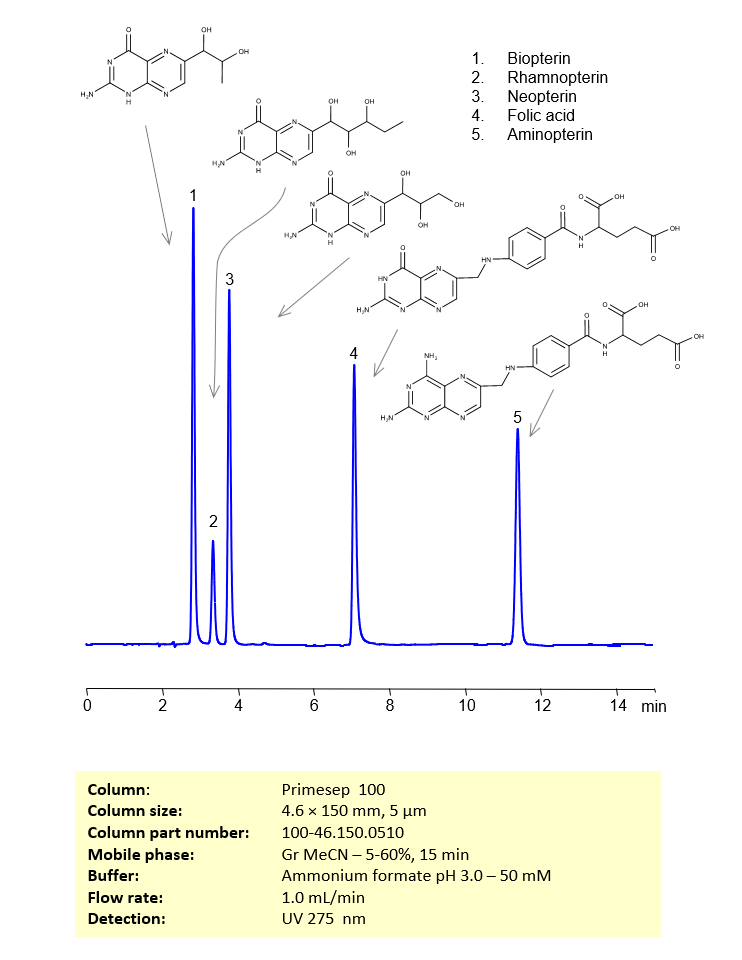
These five pterins can be retained, separated and analyzed using a reverse-phase Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and ammonium formate, which serves as a buffer. This analytical method can be monitored using UV detection at 275 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Biopterin, Rhamnopterin, Neopterin, Folic Acid, Aminopterin
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN -5-60%, 10 min |
| Buffer | Ammonium formate pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275 nm |
| Peak Retention Time | 2.21, 3.28, 3.81, 6.85, 11.21 min |
| Sample concentration | 0.02 mg/ml |
| Injection volume | 5 µl |
| Sample diluent | H2O + NaOH |
| LOD | 10 ppb |
Description
| Class of Compounds | Pteridines |
| Analyzing Compounds | Biopterin, Rhamnopterin, Neopterin, Folic Acid, Aminopterin |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Biopterin
Folic Acid
Neopterin
Rhamnopterin

HPLC Method for Separation of Folic Acid and Aminopterin on Primesep 100 Column
June 7, 2023
HPLC Method for Separation of Folic Acid and Aminopterin on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
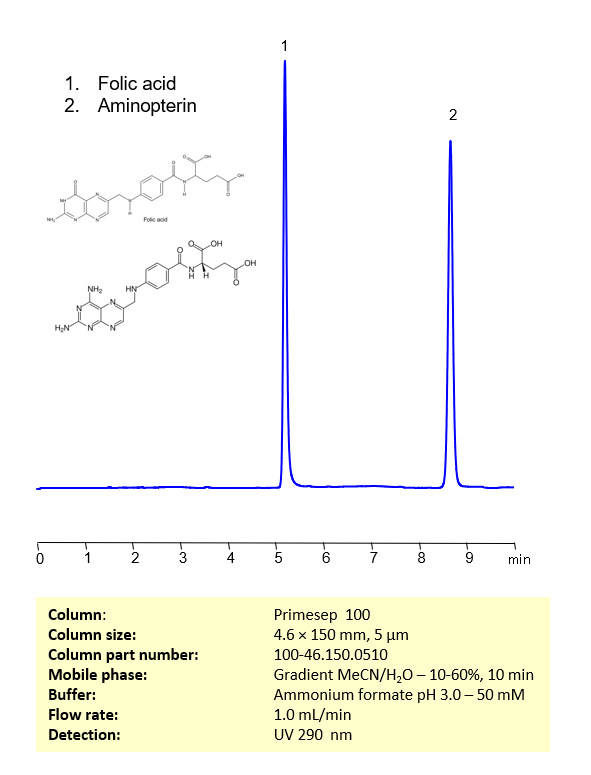
Aminopterin and folic acid are chemically related compounds that play significant roles in biochemistry and medicine.
Folic acid, also known as vitamin B9, is an essential nutrient that our bodies need to function correctly. It plays a vital role in many bodily functions, including DNA synthesis and repair, cell division and growth, and the production of red blood cells. Humans cannot synthesize folic acid and, therefore, must obtain it through their diet or via supplementation.
Aminopterin is a derivative of folic acid. It was one of the first antifolate drugs, a class of medication that inhibits the enzyme dihydrofolate reductase (DHFR). This enzyme is critical for converting folic acid into its active form, tetrahydrofolate, which is used in the synthesis of nucleotides for DNA and RNA.
By inhibiting DHFR, aminopterin interferes with the synthesis of DNA and RNA, blocking cell replication. This property made it useful as a cancer drug, as it can stop the rapid cell division characteristic of cancerous cells. However, aminopterin is highly toxic and is no longer used clinically. It has been largely replaced by less toxic and more effective antifolate drugs, such as methotrexate.
It’s important to note that while aminopterin and folic acid are chemically related, their biological effects are very different due to their interactions with the enzyme DHFR. Always consult with a healthcare provider for accurate and up-to-date information.
Aminopterin and folic acid can be retained, separated, and analyzed using a reverse-phase Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and ammonium formate, which serves as a buffer. This analytical method can be monitored using UV detection at 290 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Aminopterin, Folic Acid
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN -10-60%, 10 min |
| Buffer | Ammonium formate pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 290 nm |
| Peak Retention Time | 5.08 min, 8.32 min |
| Sample concentration | 0.02 mg/ml |
| Injection volume | 5 µl |
| Sample diluent | H2O+NaOH |
| LOD | 0.04 ppm |
Description
| Class of Compounds | Drug, Aminopterin, Antifolates, Acid |
| Analyzing Compounds | Aminopterin, Folic Acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Folic Acid

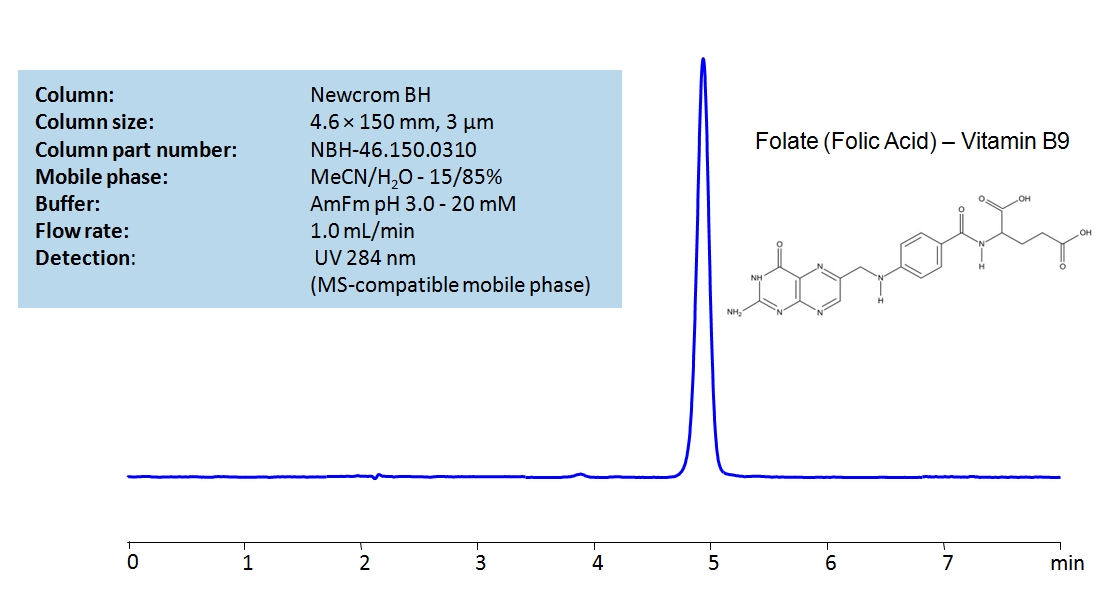
HPLC Determination of Folic Acid (Vitamin B9) on Newcrom BH Column
May 17, 2021
HPLC Method for Folic Acid on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Folic Acid.
Folic Acid, also known as vitamin B9, folic acid is crucial for many functions in the body, including DNA synthesis and repair, cell division, and growth. As an essential vitamin, Folic acid must be obtained through diet or supplementation, as humans cannot synthesize it. Vitamin B-9 is a crucial prenatal vitamin. that also helps the body use iron properly. It has the chemical formula C19H19N7O6. You can find detailed UV spectra of Folic Acid and information about its various lambda maxima by visiting the following link.
Folic acid can be retained in HPLC using a Newcrom BH mixed-mode column by using a mobile phase of acetonitrile (ACN) and water with Ammonium Formate (AmFm) as a buffer allowing the use of either UV or MS detector.
| Column | Newcrom BH, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 15/85% |
| Buffer | AmFm – pH 3.0 – 20 mm |
| Flow Rate | 1.0 ml/min |
| Detection | UV 284 nm |
| Class of Compounds |
Vitamin |
| Analyzing Compounds | Folic Acid |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended

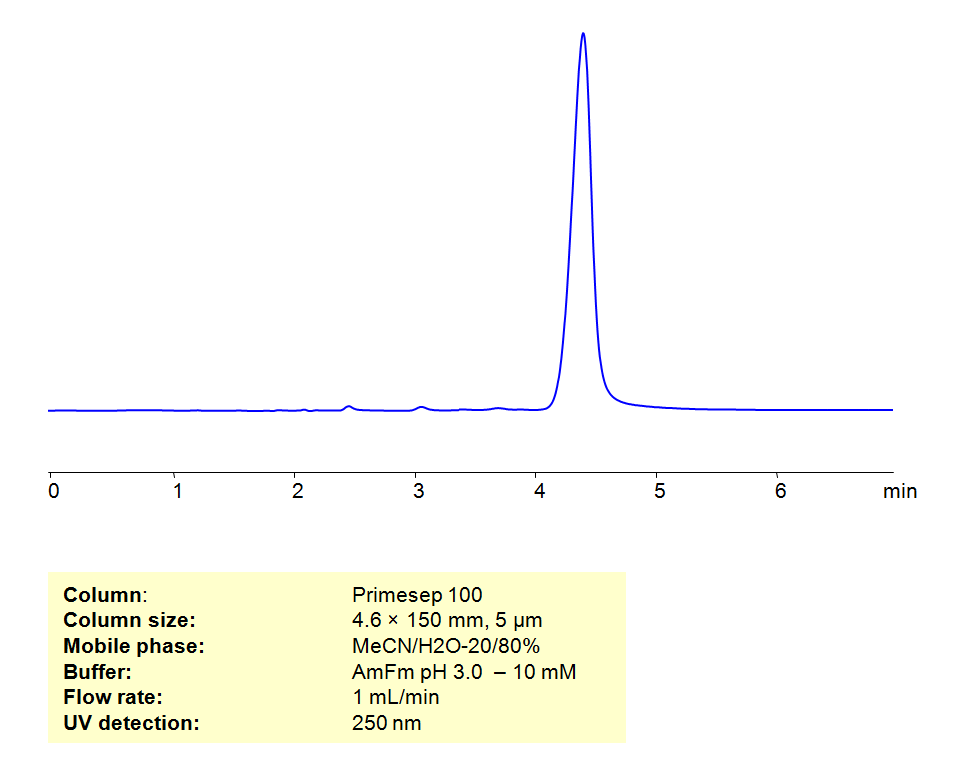
HPLC Determination of Folic Acid on Primesep 100 Column
May 20, 2019
HPLC Method for Folic Acid on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Folic Acid.
Folic acid is a synthetic form of folate, a B vitamin, that is used as in supplements and to fortify food. Folic acid can be retained using Primesep 100 mixed-mode column, which has both hydrophobic and ion exchange properties due to embedded acidic ion-pairing groups. The analytical method uses a mobile phase of acetonitrile (ACN) and water with ammonium formate (AmFm) buffer, making the method MS-compatible. Can also use UV detection at 250nm.
| Column | Primesep 100 |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | AmFm pH 3.0- 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Folic Acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

Analysis of Folic Acid and Related Impurities
July 3, 2013
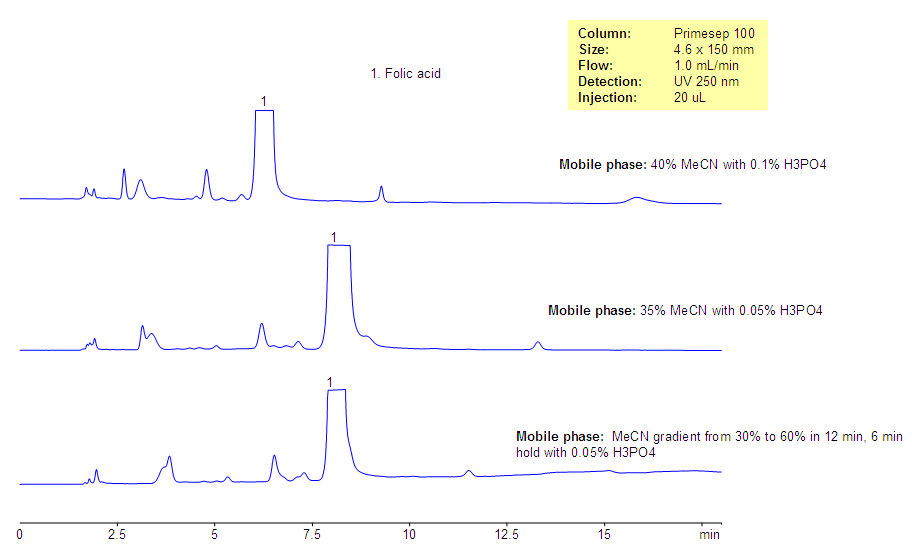
Folic acid is a water-soluble vitamin. It belongs to Group B of vitamins. Folate is composed of the aromatic pteridine ring linked to para-aminobenzoic acid and one or more glutamate residues. Folic acid (Vitamin B9) and related impurities were analyzed on the Primesep 100 mixed-mode HPLC column. This column can be used for analysis of this and other water-soluble vitamins. Several impurities and folic acid are well separated. The method is robust and can be used for analysis of folic acid in supplements and other compositions.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Folic Acid |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options