| CAS Number | 123-03-5 |
|---|---|
| Molecular Formula | C21H38ClN |
| Molecular Weight | 339.990 |
| InChI Key | YMKDRGPMQRFJGP-UHFFFAOYSA-M |
| LogP | 2.60 |
| Synonyms |
|
Applications:
UV- Vis Spectrum of Cetylpyridinium Chloride
June 17, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Cetylpyridinium Chloride check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

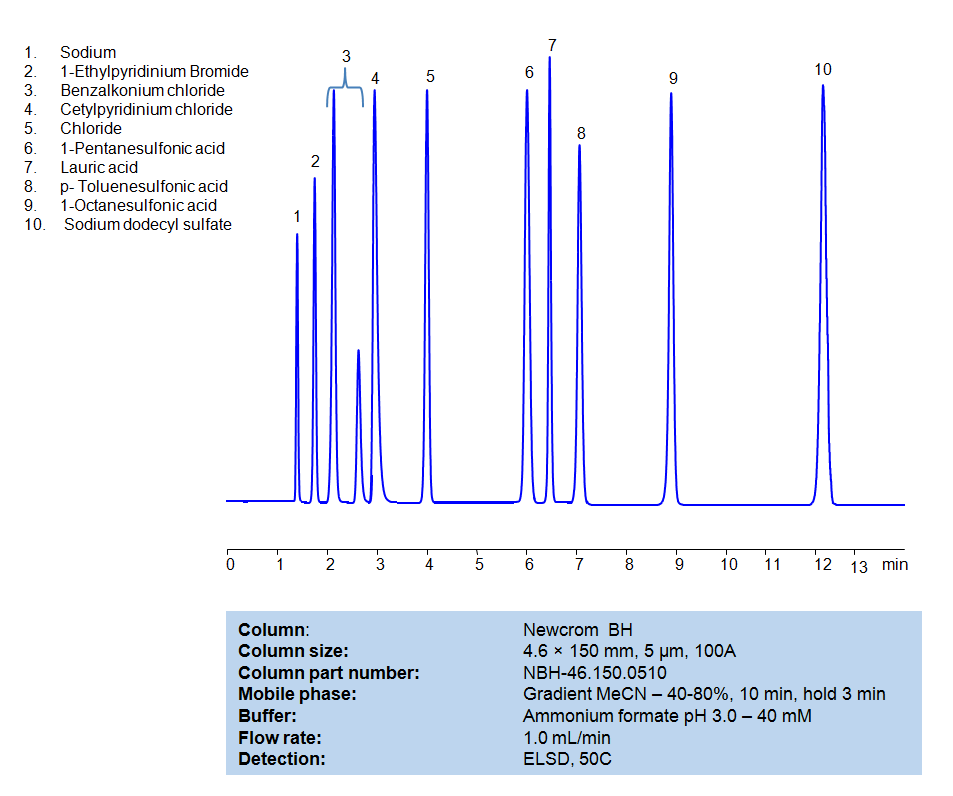
HPLC Method for Separation of Hydrophobic, Cationic and Anionic Surfactants on Newcrom BH Column
July 10, 2023
HPLC Method for Separation of Hydrophobic, Cationic and Anionic Surfactants on Newcrom BH by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Surfactants, also known as surface-active agents, are compounds that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants.
They are often classified according to the charge of the polar head group:
Anionic Surfactants: These surfactants have a negative charge on their polar head group. Common examples include soap, sodium laureth sulfate, and sodium lauryl sulfate. They are commonly used in detergents and shampoos due to their ability to emulsify oils and hold dirt in suspension, so it can be rinsed away.
Cationic Surfactants: These surfactants have a positive charge on their polar head group. Examples include cetyltrimethylammonium bromide (CTAB) and benzalkonium chloride. These are often used as antiseptics and can also be found in hair conditioners because they reduce static cling.
Nonionic Surfactants: These surfactants have no charge on their polar head group. Examples include alcohol ethoxylates, nonylphenol ethoxylates, and polysorbates. Nonionic surfactants are often used in laundry and dishwasher detergents.
All compounds can be retained, separated, and analyzed using a reverse-phase Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and Ammonium formate, which serves as a buffer. This analytical method can be detected with an Evaporative Light Scattering Detector (ELSD) or any other evaporative detection method (CAD, ESI-MS).
High Performance Liquid Chromatography (HPLC) Method for Analysis of Benzalkonium chloride, Cetylpyridinium Chloride, 1-Pentanesulfonic acid, Dodecanoic acid (Lauric acid), p-Toluenesulfonic Acid (PTSA), 1-Octanesulfonic acid, Sodium dodecyl sulfate, 1-Ethylpyridinium bromide
Condition
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN -40-80%, 10 min |
| Buffer | Ammonium formate pH 3.0 – 40 mM |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, 50C |
Description
| Class of Compounds | Surfactants |
| Analyzing Compounds | Benzalkonium chloride, Cetylpyridinium Chloride, 1-Pentanesulfonic acid, Dodecanoic acid (Lauric acid), p-Toluenesulfonic Acid (PTSA), 1-Octanesulfonic acid, Sodium dodecyl sulfate, 1-Ethylpyridinium bromide |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
1-Octanesulfonic acid
1-Pentanesulfonic acid
Benzalkonium chloride
Cetylpyridinium Chloride
Dodecanoic acid (Lauric acid)
Sodium dodecyl sulfate
p-Toluenesulfonic Acid (PTSA)

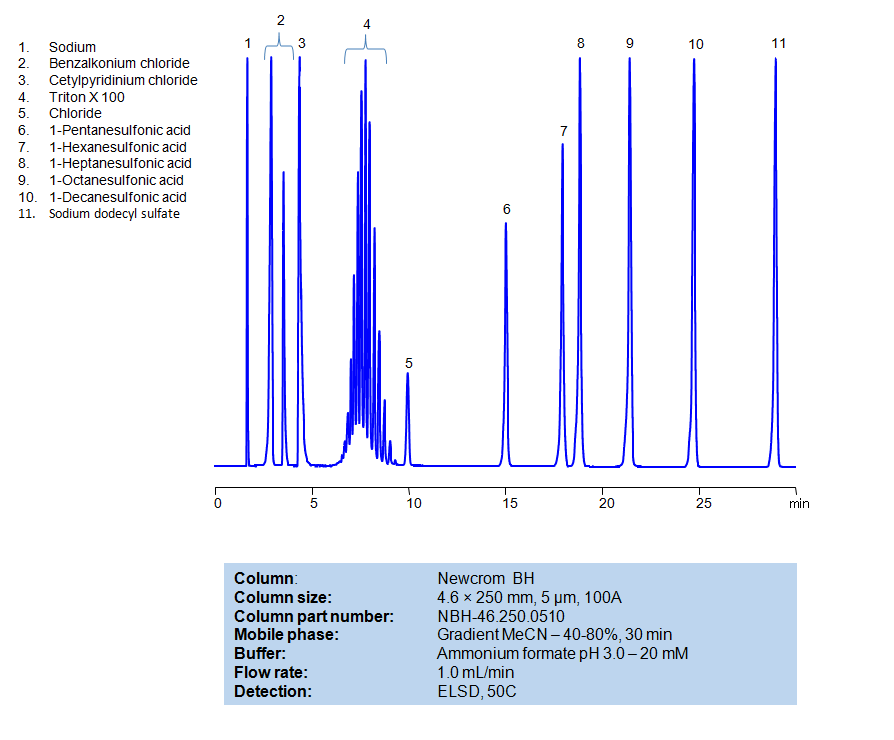
HPLC Method for Separation of Hydrophobic, Cationic, Nonionic and Anionic Surfactants on Newcrom BH Column
July 10, 2023
HPLC Method for Separation of Hydrophobic, Cationic, Nonionic and Anionic Surfactants on Newcrom BH by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Surfactants, also known as surface-active agents, are compounds that lower the surface tension (or interfacial tension) between two liquids or between a liquid and a solid. Surfactants may act as detergents, wetting agents, emulsifiers, foaming agents, or dispersants.
They are often classified according to the charge of the polar head group:
Anionic Surfactants: These surfactants have a negative charge on their polar head group. Common examples include soap, sodium laureth sulfate, and sodium lauryl sulfate. They are commonly used in detergents and shampoos due to their ability to emulsify oils and hold dirt in suspension, so it can be rinsed away.
Cationic Surfactants: These surfactants have a positive charge on their polar head group. Examples include cetyltrimethylammonium bromide (CTAB) and benzalkonium chloride. These are often used as antiseptics and can also be found in hair conditioners because they reduce static cling.
Nonionic Surfactants: These surfactants have no charge on their polar head group. Examples include alcohol ethoxylates, nonylphenol ethoxylates, and polysorbates. Nonionic surfactants are often used in laundry and dishwasher detergents.
All compounds can be retained, separated, and analyzed using a reverse-phase Newcrom BH, 4.6 x 250 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and Ammonium formate, which serves as a buffer. This analytical method can be detected with an Evaporative Light Scattering Detector (ELSD) or any other evaporative detection method (CAD, ESI-MS).
High Performance Liquid Chromatography (HPLC) Method for Analysis of Benzalkonium chloride, Cetylpyridinium Chloride, Triton X100, 1-Pentanesulfonic acid, 1-Hexanesulfonic acid, sodium salt, 1-Heptanesulfonic acid, 1-Decanesulfonic acid, Sodium dodecyl sulfate, 1-Octanesulfonic acid
Condition
| Column | Newcrom BH, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN -40-80%, 30 min |
| Buffer | Ammonium formate pH 3.0 – 20 mM |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, 50C |
Description
| Class of Compounds | Aliphatic sulfonic acid |
| Analyzing Compounds | Benzalkonium chloride, Cetylpyridinium Chloride, Triton X100, 1-Pentanesulfonic acid, 1-Hexanesulfonic acid, sodium salt, 1-Heptanesulfonic acid, 1-Decanesulfonic acid, Sodium dodecyl sulfate, 1-Octanesulfonic acid |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
1-Heptanesulfonic acid
1-Hexanesulfonic acid, sodium salt
1-Octanesulfonic acid
1-Pentanesulfonic acid
Benzalkonium chloride
Cetylpyridinium Chloride
Sodium dodecyl sulfate
Triton X100

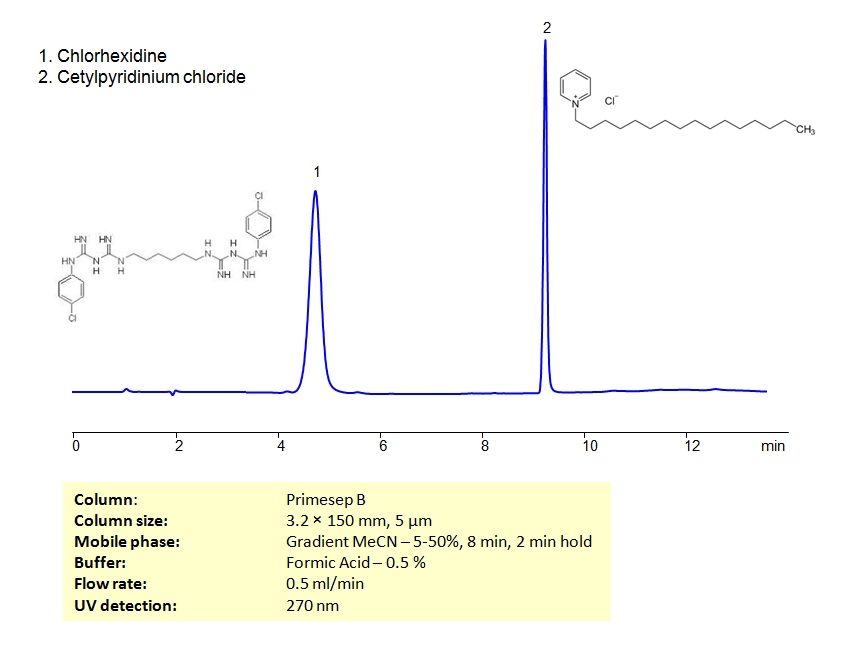
HPLC Method for Separation of Chlorhexidine and Cetylpyridinium Chloride on Primesep B Column
January 22, 2020
HPLC Method for Cetylpyridinium Chloride, Chlorhexidine on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cetylpyridinium Chloride, Chlorhexidine.
Chlorhexidine gluconate, or simply chlorhexidine, is a biguanide used as an antiseptic and disinfectant. It is a component of mouthwash rinses that has been shown to reduce plaque, gingivitis and oral bacteria. It’s also used as a topical agent for skin disinfection. Cetylpyridinium chloride is another type of antiseptic used in mouthwash rinses. Both compounds are cationic.
You can find detailed UV spectra of Chlorhexidine and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Cetylpyridinium Chloride and information about its various lambda maxima by visiting the following link.
Cetylpyridinium Chloride, Chlorhexidine can be separated using HPLC on SIELC’s reverse-phase (RP) mixed-mode Primesep B column with the mobile phase of acetonitrile (ACN) and water with formic acid buffer and UV detected at 270nm.
| Column | Primesep B, 3.2 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O |
| Buffer | Formic Acid – 0.5% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Surfactant, Hydrophobic, Ionizable |
| Analyzing Compounds | Cetylpyridinium Chloride, Chlorhexidine |
Application Column
Primesep B
Column Diameter: 3.2 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Chlorhexidine

HPLC Method for Analysis of Cetylpyridinium Chloride and Triethylene Glycol on Obelisc N Column
July 8, 2011
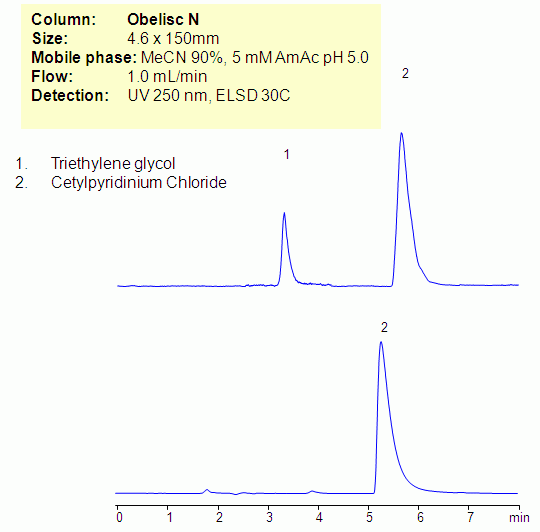
Cetylpyridinium chloride is hydrophobic basic compound and triethylene glycol is hydrophilic neutral compounds. Quantitative analysis of both compounds is problematic due to a different nature of these two analytes. Both compounds were analyzed on an Obelisc N column in HILIC/cation-exchange mode. Cetylpyridinium chloride is retained by cation-exchange mechanism, and triethylene glycol is retained by HILIC mechanism. Mixed-mode HILIC approach allows to retain compounds either based on multiple or single mechanisms interaction, thus providing a valuable approach for analysis. Cetylpyridinium chloride and triethylene glycol can be monitored by combination of UV and ELSD/CAD.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc ph 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250nm, ELSD |
| Class of Compounds |
Surfactant, Hydrophobic, Ionizable |
| Analyzing Compounds | Triethylene glycol, Cetylpyridinium Chloride |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsTriethylene Glycol
UV Detection

HPLC Method for Analysis of Cetylpyridinium Chloride on Primesep D Column
November 21, 2006
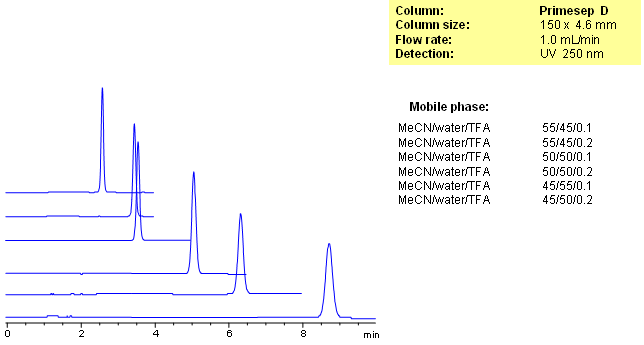
Cetylpyridinium Chloride is an antiseptic agent used in mouthwash or dental care for oral and throat care. Primesep D retains cetylpyridinium by a combination of reversed-phase and ion-exchange mechanisms. The anion-exchange properties of the column can be adjusted by the TFA concentration in the mobile phase and the reversed-phase properties are adjusted by the percentage of acetontrile. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) and UV detection at 250 nm.
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
HPLC Separation of Surfactants
November 21, 2006
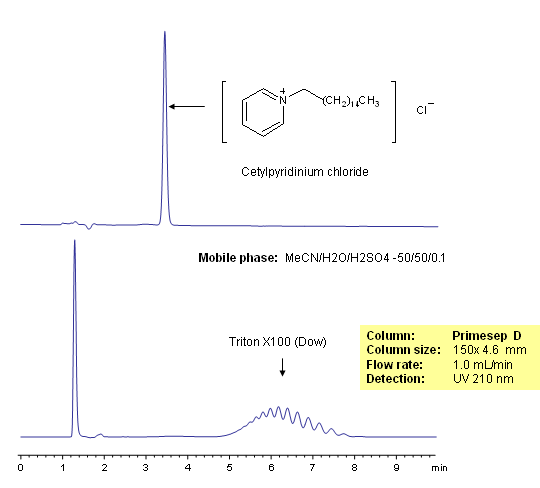
Surfactants are molecules that contain both hydrophilic and hydrophobic groups, usually in the form of a hydrophilic head and a hydrophobic tail. Surfactants are used in detergents where they can form micelles around hydrophobic dirt molecules and wash them away. Triton X-100 is a surfactant with a hydrophilic polyethylene oxide chain that can be separated on a Primesep D reverse-phase HPLC column based on the number of oxide units in the chain. The mobile phase is water, acetonitrile (MeCN, ACN) and sulfuric acid as buffer. UV detection at 210nm.
| Column | Primesep D, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H2SO4- 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210nm |
| Class of Compounds |
Surfactant, Hydrophobic, Ionizable |
| Analyzing Compounds | Triton X-100, Cetylpyridinium Chloride |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTriton X100

Cetylpyridinium Methods with Good Efficiency and Peak Symmetry
February 11, 2004
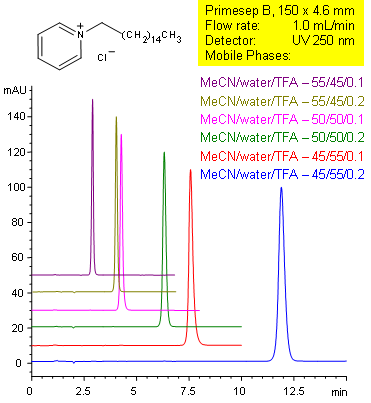
Primesep B separates tertiary amines, such as cetylpyridinium with symmetrical peak shape by a combination of reversed-phase and ion-exclusion mechanisms. The embedded basic functional group on the stationary phase shields the underlying silanols to prevent peak tailing. Retention time can be changed by changing either organic content or acid content in the mobile phase. C18 reversed-phase columns do not typically show this tuning ability with acid content. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 250 nm.
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210nm |
| Class of Compounds |
Surfactant, Hydrophobic, Ionizable |
| Analyzing Compounds | Cetylpyridinium Chloride |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCetylpyridinium Chloride
Pyridinium Ion
Quaternary Amines