| CAS Number | 55-56-1 |
|---|---|
| Molecular Formula | C22H30Cl2N10 |
| Molecular Weight | 505.451 |
| InChI Key | GHXZTYHSJHQHIJ-UHFFFAOYSA-N |
| LogP | 0.08 |
| Synonyms |
|
Applications:
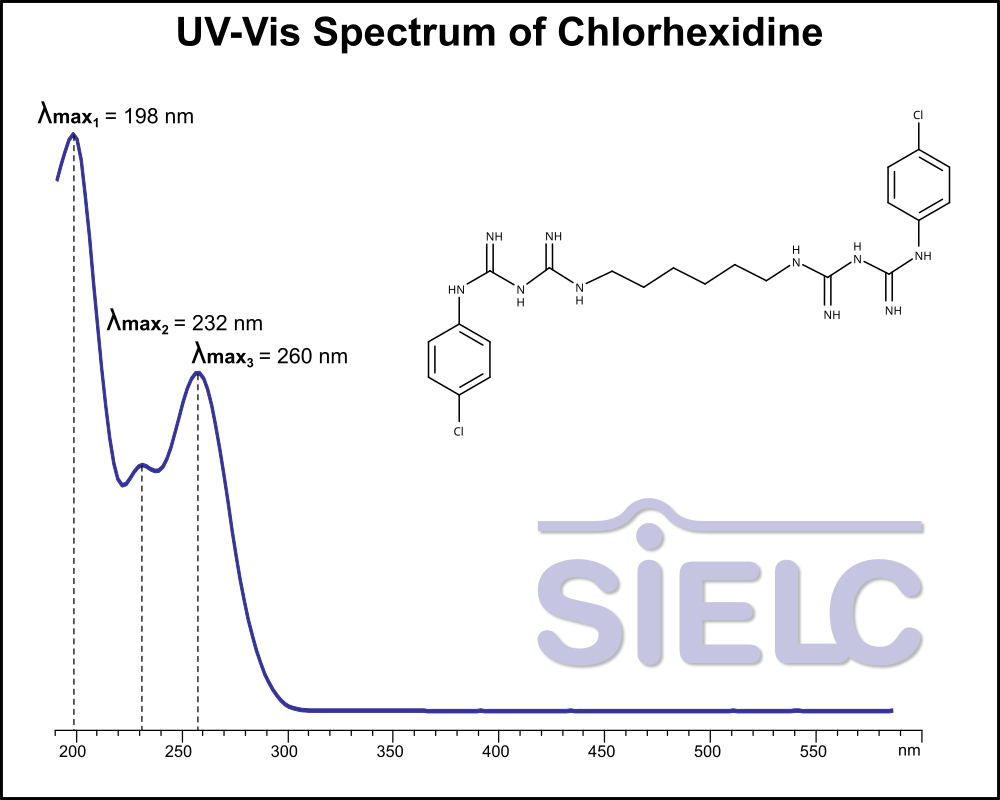
UV-Vis Spectrum of Chlorhexidine
August 4, 2025
If you are looking for optimized HPLC method to analyze Chlorhexidine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of Chlorhexidine in Biofluids: Blood Plasma and Urine on BIST B+ Column
November 30, 2022
HPLC Method for Analysis of Chlorhexidine in Biofluids: Blood Plasma and Urine on BIST B+ by SIELC Technologies.
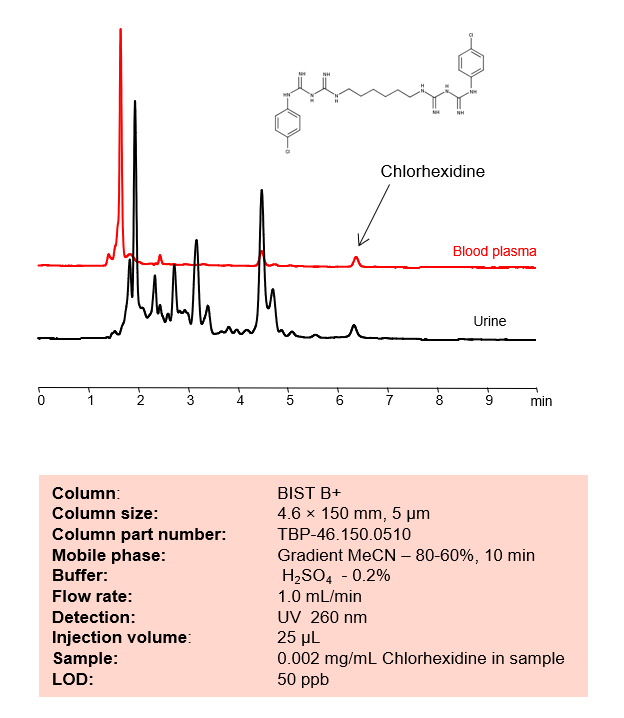
Chlorhexidine is a common antiseptic used intensively in hand sanitizers and other antibacterial products. It has the chemical formula C22H30Cl2N10. It is primarily used for skin surgical instrument disinfection. Despite being used in mouthwash, one of the most common side effects is tooth discoloration. You can find detailed UV spectra of Chlorhexidine and information about its various lambda maxima by visiting the following link.
Typical HPLC methods used to analyze this product are done in reverse phase (RP) mode, but this is complicated by the presence of two basic groups which, with the overall hydrophobic characteristics of the molecule, usually produce asymmetrical peaks.
Chlorhexidine can be retained and analyzed using the BIST B+ stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
Condition
| Column | BIST B+, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time | 6.2 min |
Description
| Class of Compounds | Topical antiseptic |
| Analyzing Compounds | Chlorhexidine |
Sample preparation:
| Plasma/urine sample was spiked with a small amount of Chlorhexidine and then diluted 5 times with acetonitrile. A formed precipitate was removed by centrifugation and the cleared solution was used directly for injection. |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method for Analysis of Chlorhexidine on BIST B+ Column
November 30, 2022
HPLC Method for Chlorhexidine on BIST B+ by SIELC Technologies
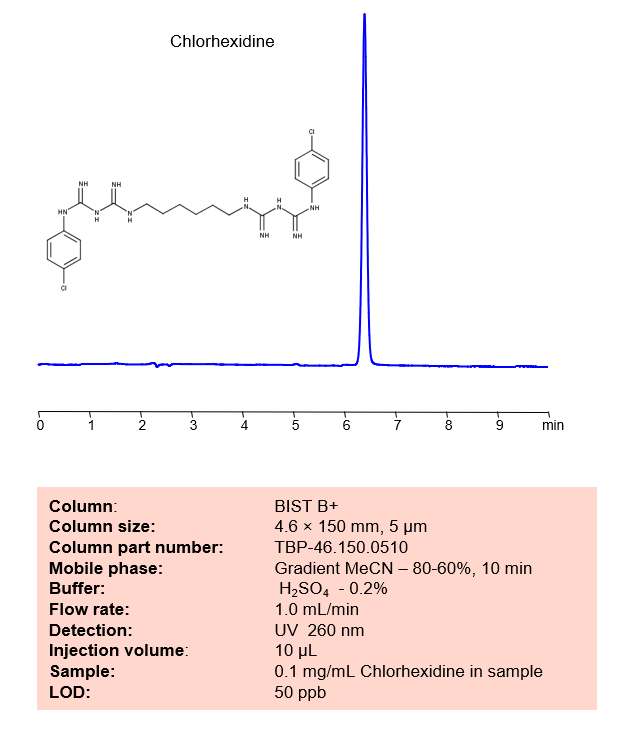
Chlorhexidine is a common antiseptic used intensively in hand sanitizers and other antibacterial products. It has the chemical formula C22H30Cl2N10. It is primarily used for skin surgical instrument disinfection. Despite being used in mouthwash, one of the most common side effects is tooth discoloration. You can find detailed UV spectra of Chlorhexidine and information about its various lambda maxima by visiting the following link.
Typical HPLC methods used to analyze this product are done in reverse phase (RP) mode, but this is complicated by the presence of two basic groups which, with the overall hydrophobic characteristics of the molecule, usually produce asymmetrical peaks.
Chlorhexidine can be retained and analyzed using the BIST B+ stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a [buffer] buffer. Detection is performed using UV.
Condition
| Column | BIST B+, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time | 6.2 min |
Description
| Class of Compounds | Topical antiseptic |
| Analyzing Compounds | Chlorhexidine |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

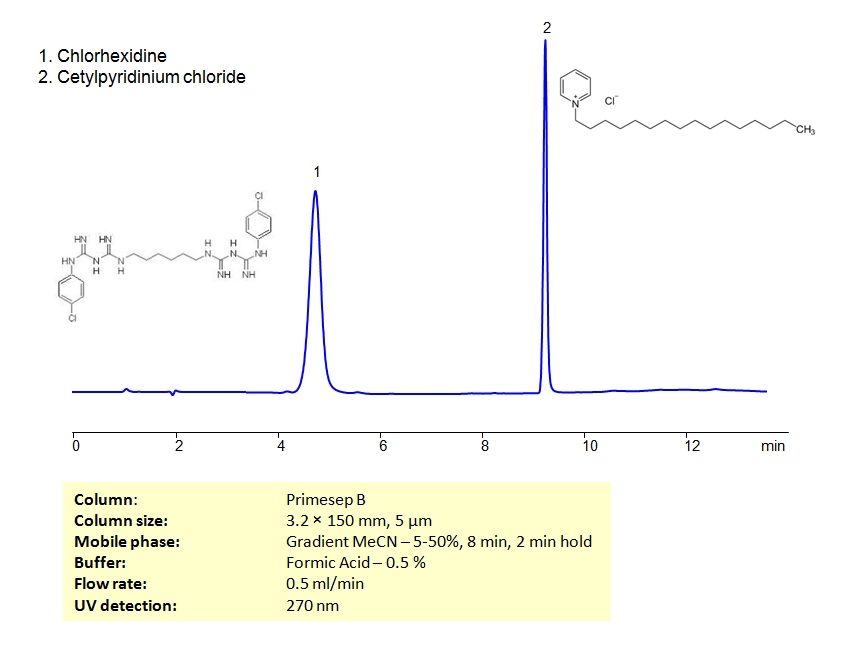
HPLC Method for Separation of Chlorhexidine and Cetylpyridinium Chloride on Primesep B Column
January 22, 2020
HPLC Method for Cetylpyridinium Chloride, Chlorhexidine on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cetylpyridinium Chloride, Chlorhexidine.
Chlorhexidine gluconate, or simply chlorhexidine, is a biguanide used as an antiseptic and disinfectant. It is a component of mouthwash rinses that has been shown to reduce plaque, gingivitis and oral bacteria. It’s also used as a topical agent for skin disinfection. Cetylpyridinium chloride is another type of antiseptic used in mouthwash rinses. Both compounds are cationic.
You can find detailed UV spectra of Chlorhexidine and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Cetylpyridinium Chloride and information about its various lambda maxima by visiting the following link.
Cetylpyridinium Chloride, Chlorhexidine can be separated using HPLC on SIELC’s reverse-phase (RP) mixed-mode Primesep B column with the mobile phase of acetonitrile (ACN) and water with formic acid buffer and UV detected at 270nm.
| Column | Primesep B, 3.2 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O |
| Buffer | Formic Acid – 0.5% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Surfactant, Hydrophobic, Ionizable |
| Analyzing Compounds | Cetylpyridinium Chloride, Chlorhexidine |
Application Column
Primesep B
Column Diameter: 3.2 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Chlorhexidine

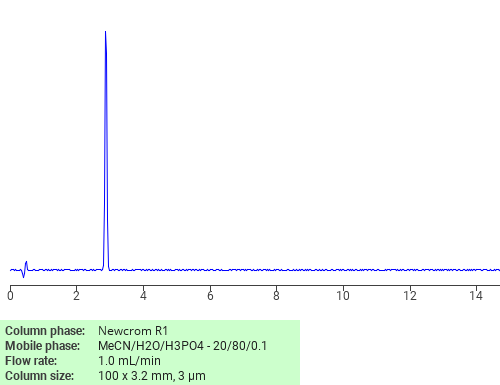
Separation of Chlorhexidine on Newcrom R1 HPLC column
February 16, 2018
Chlorhexidine can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options