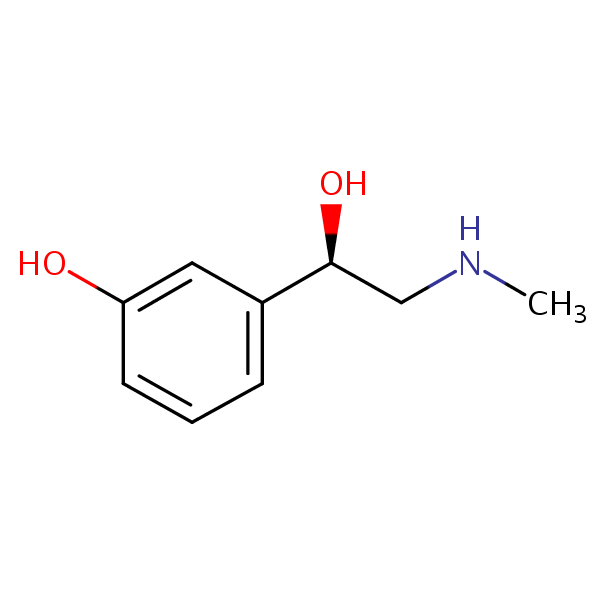
| CAS Number | 59-42-7 |
|---|---|
| Molecular Formula | C9H13NO2 |
| Molecular Weight | 167.208 |
| InChI Key | SONNWYBIRXJNDC-VIFPVBQESA-N |
| LogP | -0.310 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Neurotransmitters Phenylephrine, Epinephrine and Norepinephrine on BIST™B+ Column
July 14, 2022
HPLC Method for Phenylephrine hydrochloride, Phenylephrine, Norphenylephrine, Epinephrine on BIST B+ by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Phenylephrine hydrochloride, Phenylephrine, Norphenylephrine, Epinephrine.
Epinephrine, also known as Adrenaline, is a hormone with the chemical formula C9H13NO3. Exercise is one of the main stimulants of epinephrine, but it is epinephrine which has an effect on contributing to stronger emotions and enhancing long-term memory. As a hormone, it is involved in regulating involuntary operations of nearly all internal organs. As a medication, it is used to treat allergic reaction anaphylaxis and cardiac arrest. When other treatments are not effective, it is occasionally used to treat asthma. The name, adrenaline, comes adrenal glands, from which it was extracted and purified. You can find detailed UV spectra of Epinephrine and information about its various lambda maxima by visiting the following link.
Norepinephrine, also known as noradrenaline and noradrenalin, is a catecholamine with the chemical formula C8H11NO3. It functions as a hormone, neurotransmitter, and neuromodulator responsible for improving alertness, focus, memory, and regulating mood. You can find detailed UV spectra of Norepinephrine and information about its various lambda maxima by visiting the following link.
Phenylephrine is a common over-the-counter decongestant, but can also be used for pupil dilation and hemorrhoid treatment. It’s chemical formula is C9H13NO2. It is sold under many brand names including Mucinex, Sudafed PE, Sinex, and many other generic brands. While it is best known as a decongestant, it has a wide variety of other pharmaceutical applications, including pupil dilation, hypotension (low blood pressure) treatment, and hemorrhoid relief.
Using SIELC’s newly introduced BIST™ method, these three similar neurotransmitters, which protonate in water, can be retained on a positively-charged anion-exchange BIST™ B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively-charged amine analytes to the positively-charged column surface and 2) a mobile phase consisting mostly of organic solvent to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Epinephrine, norepinephrine, and phenylephrine can be retained and UV detected at 200 nm.
| Column | BIST B+ |
| Mobile Phase | MeCN -80% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds |
Drug, Neurotransmitter, Catecholamine |
| Analyzing Compounds | Phenylephrine hydrochloride, Phenylephrine, Norphenylephrine, Epinephrine |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Norphenylephrine
Phenylephrine
Phenylephrine hydrochloride

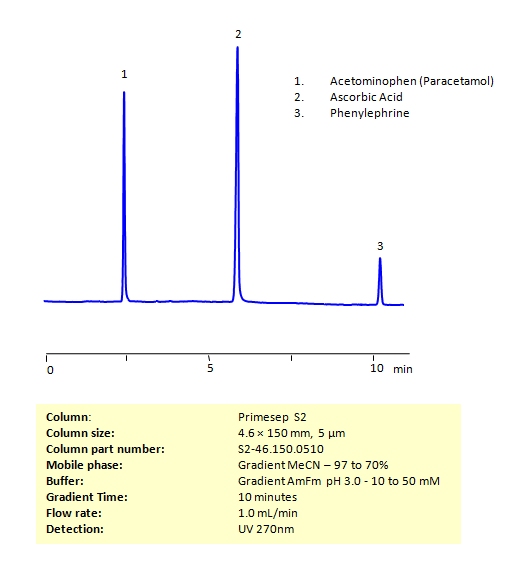
HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S2 Column
October 5, 2021
HPLC Method for Acetaminophen (Paracetamol), Phenylephrine, Phenylephrine hydrochloride, Ascorbic Acid on Primesep S2 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
Acetaminophen (or paracetamol) is one of the most popular over-the-counter painkillers all over the world (in the US it is best known under the brand name Tylenol). Ascorbic acid (also known as Vitamin C) helps your body build up muscle, cartilage, and other important building blocks in your body. Phenylephrine is a common over-the-counter decongestant, but can also be used for pupil dilation and hemorrhoid treatment. While all of these compounds are available at your local pharmacy, Vitamin C intake can reduce the body’s ability to break down acetaminophen.
You can find detailed UV spectra of Acetaminophen and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Ascorbic Acid and information about its various lambda maxima by visiting the following link.
All three of these compounds can be measured at low UV. Using a Primsep S normal phase column and a mobile phase consisting of water and acetonitrile (MeCN) with an Ammonium acetate (AmAc) buffer, these three compounds can be separated and UV detected at 270 nm. Varying the buffer concentration changes the order in which phenylephrine and ascorbic acid are retained. This method is compatible with Mass Spectrometry.
| Column | Primesep S2, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 97 to 70% |
| Buffer | Gradient Ammonium Formate pH 3.0 – 10 to 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm MS- compatible mobile phase |
| Class of Compounds | Drug, Acid |
| Analyzing Compounds | Acetaminophen (Paracetamol), Phenylephrine, Phenylephrine hydrochloride, Ascorbic Acid |
Application Column
Primesep S2
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Ascorbic Acid
Phenylephrine
Phenylephrine hydrochloride

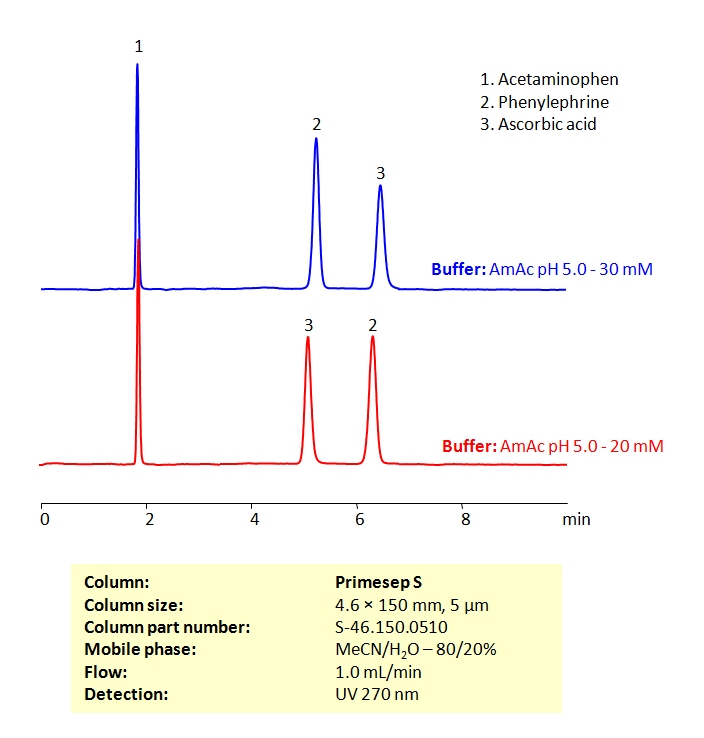
HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S Column
October 5, 2021
HPLC Method for Acetaminophen (Paracetamol), Phenylephrine, Ascorbic Acid, Phenylephrine hydrochloride on Primesep S by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
Acetaminophen, also known as Paracetamol, is a medication with the molecular formula C8H9NO2. It is an over-the-counter pain killer that also reduces fever. Unlike Ibuprofen and naproxen, it is not an anti-inflammatory drug.
Phenylephrine is a common over-the-counter decongestant, but can also be used for pupil dilation and hemorrhoid treatment. It’s chemical formula is C9H13NO2. It is sold under many brand names including Mucinex, Sudafed PE, Sinex, and many other generic brands. While it is best known as a decongestant, it has a wide variety of other pharmaceutical applications, including pupil dilation, hypotension (low blood pressure) treatment, and hemorrhoid relief.
Ascorbic Acid is a vitamin with the molecular formula C6H8O6. Typically, it is used to treat scurvy, support immune system, and preserve food. It is a white to light yellow powder that is easily dissolved in water. It can be found in a large variety of fruits and vegetables, especially in citrus.
You can find detailed UV spectra of Acetaminophen and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Ascorbic Acid and information about its various lambda maxima by visiting the following link.
Acetaminophen (Paracetamol), Phenylephrine, Ascorbic Acid, Phenylephrine hydrochloride can be retained and analyzed using the Primesep S stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with an ammonium acetate buffer. Detection is performed using UV.
| Column | Primesep S, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | Ammonium acetate pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270nm MS- compatible mobile phase |
| Class of Compounds | Drug |
| Analyzing Compounds | Acetaminophen (Paracetamol), Phenylephrine, Ascorbic Acid, Phenylephrine hydrochloride |
Application Column
Primesep S
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Ascorbic Acid
Phenylephrine
Phenylephrine hydrochloride

HPLC Separation of Pseudoephedrine, Norephedrine, Phenylephrine, and Norphenylephrine Using Hydrogen Bonding Mode
June 15, 2012
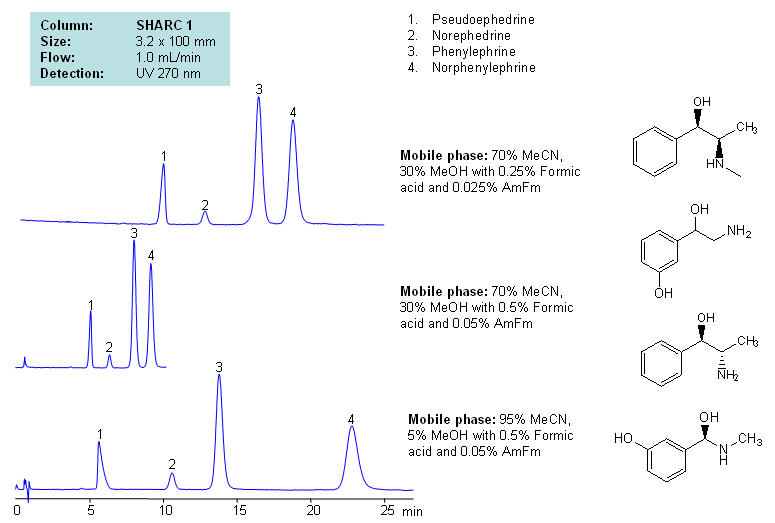
Application Notes: Neurotransmitters are polar basic compounds. Several HPLC techniques are used to analyze these hydrophilic molecules. The most common techniques are reversed-phase with ion-pairing reagents chromatography, ion chromatography, HILIC, and mixed-mode chromatography. SIELC developed a new approach of analysis, which is based on hydrogen bond interactions. Hydrogen-bonding offers unique and alternative selectivity to traditional approaches like reversed-phase, HILIC, ion-exchange, and mixed-mode chromatography. Our methods are fully compatible with ELSD, LC/MS and prep chromatography.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A. To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application compounds: Pseudoephedrine, norephedrine, phenylephrine and norphenylephrine
Detection technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Pseudoephedrine, Norephedrine, Phenylephrine, Norphenylephrine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsNorphenylephrine
Phenylephrine
Pseudoephedrine (PSE)

HPLC Separation of Phenylephrine with Tannic Acid Formulation
November 21, 2010
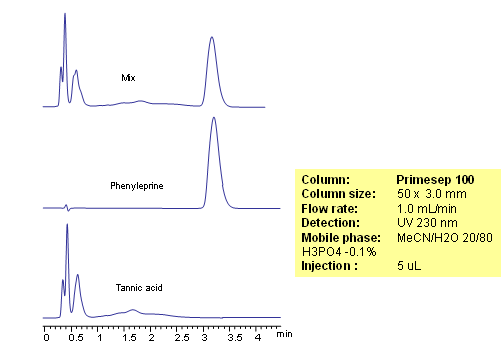
Phenylephrine is a hydrophilic basic compound. Phenylephrine is used primarily as a decongestant in cough composition. Tannic acid is poly-phenolic compound. Tannic acid is also used to produce tannate salts of certain antihistamins and anti-tussives to impart increased stability or slow release properties to the API (active pharmaceutical ingredient). Phenylephrine in the presence of tannic acid is analyzed by mixed-mode HPLC on a Primesep 100 column. Retention time for phenylephrine is controlled by the amount of acetonitrile and buffer concentration. Tannic acid elutes closer to void and not interferes with analysis of phenylephrine. Method can be used with UV detection.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTannic Acid

HPLC Separation of Caffeine and Phenylephrine on Primesep 200
October 14, 2010
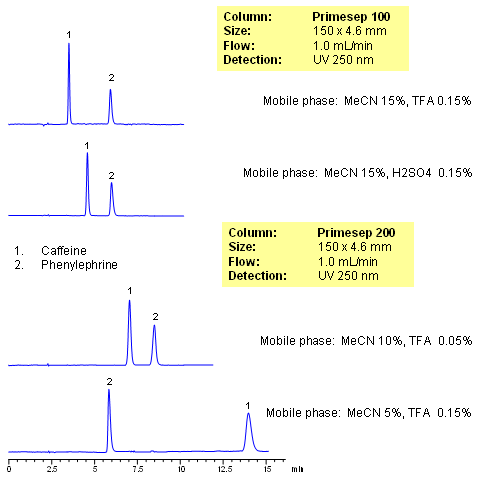
Caffeine and phenylephrine are some of the components of various pain killer/fever reducers/cough compositions. In this application, both of these compounds are separated on Primesep 100 and Primesep 200 columns. Caffeine is retained by reversed-phase mechanisms and phenylephrine is retained by combination of reversed-phase and cation-exchange mechanisms. Retention time for caffeine is controlled by the amount of acetonitrile, while retention time of phenylephrine is controlled by amount of the acetonitrile, buffer concentration and buffer pH. The method can be used for analysis of components of pain, cough and cold medication in pharmaceutical production. Method is robust and reproducible and provides good retention and peak shape. Compounds are monitored by UV, ELSD, CAD or LC/MS. Analysis of active components on biofluids (urine, plasma, blood, etc) is possible with additional sample preparation (protein precipitation, SPE, etc.)
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Phenylephrine

HPLC Separation of Caffeine and Phenylephrine on Primesep 100
October 14, 2010

Caffeine and phenylephrine are some of the components of various pain killer/fever reducers/cough compositions. In this application, both of these compounds are separated on Primesep 100 and Primesep 200 columns. Caffeine is retained by reversed-phase mechanisms and phenylephrine is retained by combination of reversed-phase and cation-exchange mechanisms. Retention time for caffeine is controlled by the amount of acetonitrile, while retention time of phenylephrine is controlled by amount of the acetonitrile, buffer concentration and buffer pH. The method can be used for analysis of components of pain, cough and cold medication in pharmaceutical production. Method is robust and reproducible and provides good retention and peak shape. Compounds are monitored by UV, ELSD, CAD or LC/MS. Analysis of active components on biofluids (urine, plasma, blood, etc) is possible with additional sample preparation (protein precipitation, SPE, etc.)
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Phenylephrine

HPLC Separation of Polar Drugs with MS-compatible Method
October 12, 2010
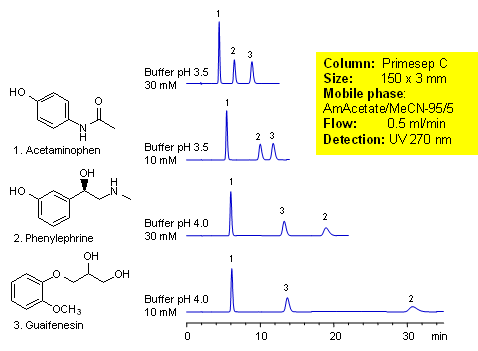
Acetaminophen is an over-the-counter pain and fever reducer, and a major component of cold and flue remedies. Phenylephrine is a decongestant. Guaifenesin is a mucolytic agent used to relieve respiratory difficulties. These three compounds of cough medication were separated by mixed-mode chromatography on a Primesep C HPLC column. Retention and order of elution for phenylephrine can be changed by buffer concentration and buffer pH. Method can be used for analysis of cough and cold composition during the production and in QC/QA environment. Method is compatible with LC/MS and can be used to analyze these components in biological fluids. This generic HPLC method is robust and reproducible.
| Column | Primesep C, 3×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | AmAc |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Acetaminophen, Pseudoephedrine, Guaifenesin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGuaifenesin
Phenylephrine

Effect of pH on Retention of Basic Compounds on Primesep C Columns
January 13, 2010
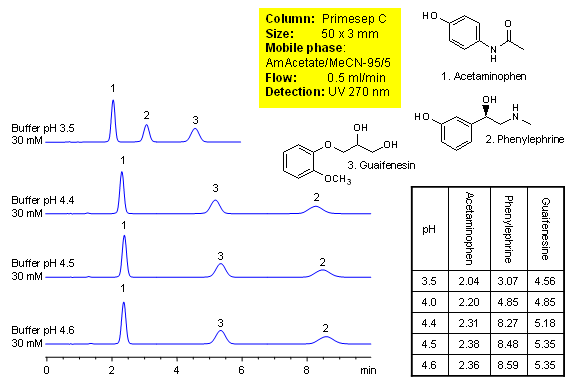
Method for separation of components of cough medication shows how retention for acetaminophen, phenylephrine and guaifenesin is controlled. Order of elution for compounds can be changed based on composition of the mobile phase. The method is compatible with UV, ELSD and LC/MS and can be used for quantitation of drugs in formulation. Primesep C mixed-mode HPLC column is ideal candidate for analysis of cough compositions. Basic compounds are well retained in mixed-mode chromatography without use of ion-pairing reagents. Several companies validated this approach. The method is reproducible and robust and can be used in both production environments and R&D. This HPLC method can be adopted as general approach for analysis of basic compounds in various mixtures.
| Column | Primesep C, 3×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | AmAc |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Acetaminophen, Pseudoephedrine, Guaifenesin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGuaifenesin
Phenylephrine

HPLC Separation of Neurotransmitters and Related Drugs
August 22, 2008
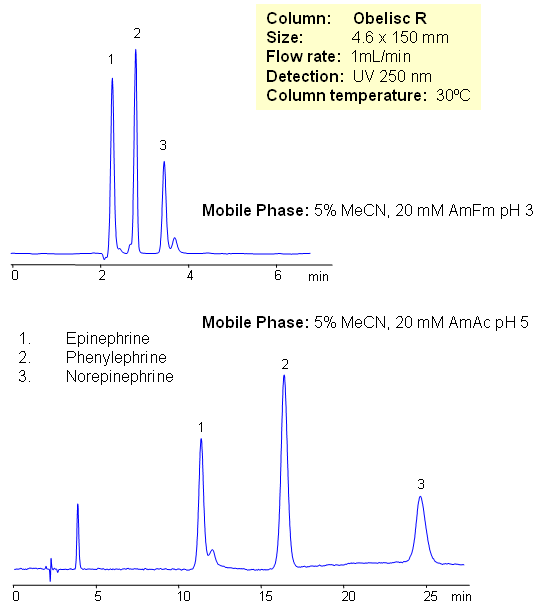
Epinephrine and norepinephrine (adrenaline and noradrenaline) are hormones and neurotransmitters. Epinephrine is synthesized from norepinephrine in a synthetic pathway shared by all catecholamines, including L-dopa, dopamine, norepinephrine, and epinephrine. Phenylephrine is used as a decongestant, available as an oral medicine or as a nasal spray. Phenylephrine is now the most common over-the-counter (OTC) decongestant. All three compounds are used in various drug compositions. Separation of epinephrine and norepinephrine is a challenging task due to polarity and close properties of two compounds. Epinephrine, norepinephrine and phenylephrine are separated in this method on Obelisc R mixed-mode HPLC columns. The method is very sensitive to variation of pH and pH adjustment can be used to achieve desired selectivity and retention time. Other catecholamines can be analyzed using this HPLC method. The method can be used as a stability indicating or a impurity profiling approach to the analysis of neurotransmitters in drug formulation, blood, serum and urine.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Phenylalanine, Norepinephrine, Epinephrine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsNorepinephrine
Phenylephrine

HPLC Separation of Acetaminophen, Phenylephrine, and Diphenhydramine
March 3, 2006
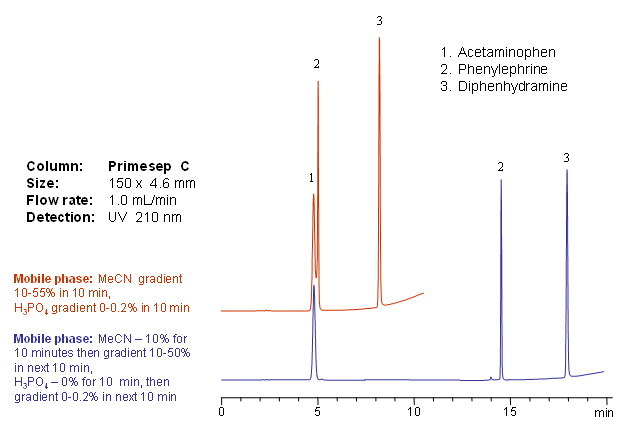
Primesep C separates acetaminophen (paracetamol), phenylephrine, and diphenhydramine– common over-the-counter cold and flu medication ingredients such as Tylenol products—by either an acetonitrile or phosphoric acid gradient. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN), and phosphoric acid with ultraviolet (UV) detection at 210 nm.
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Acetaminophen, Pseudoephedrine, Diphenhydramine |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDiphenhydramine
Phenylephrine