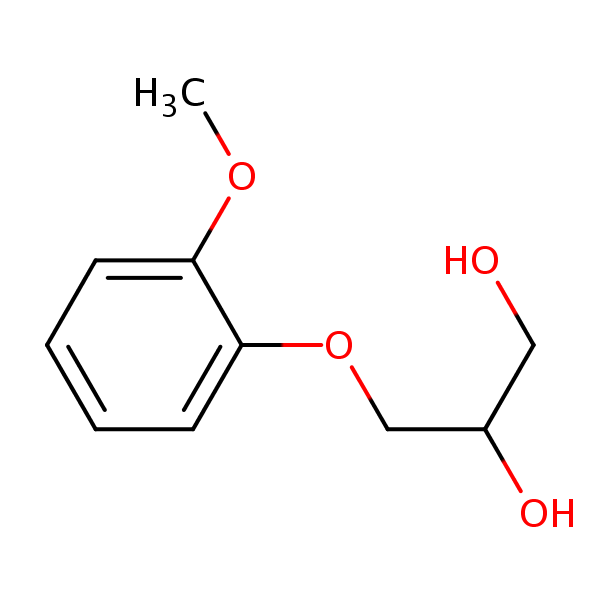
| CAS Number | 93-14-1 |
|---|---|
| Molecular Formula | C10H14O4 |
| Molecular Weight | 198.219 |
| InChI Key | HSRJKNPTNIJEKV-UHFFFAOYSA-N |
| LogP | 1.39 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Guaifenesin
July 11, 2025
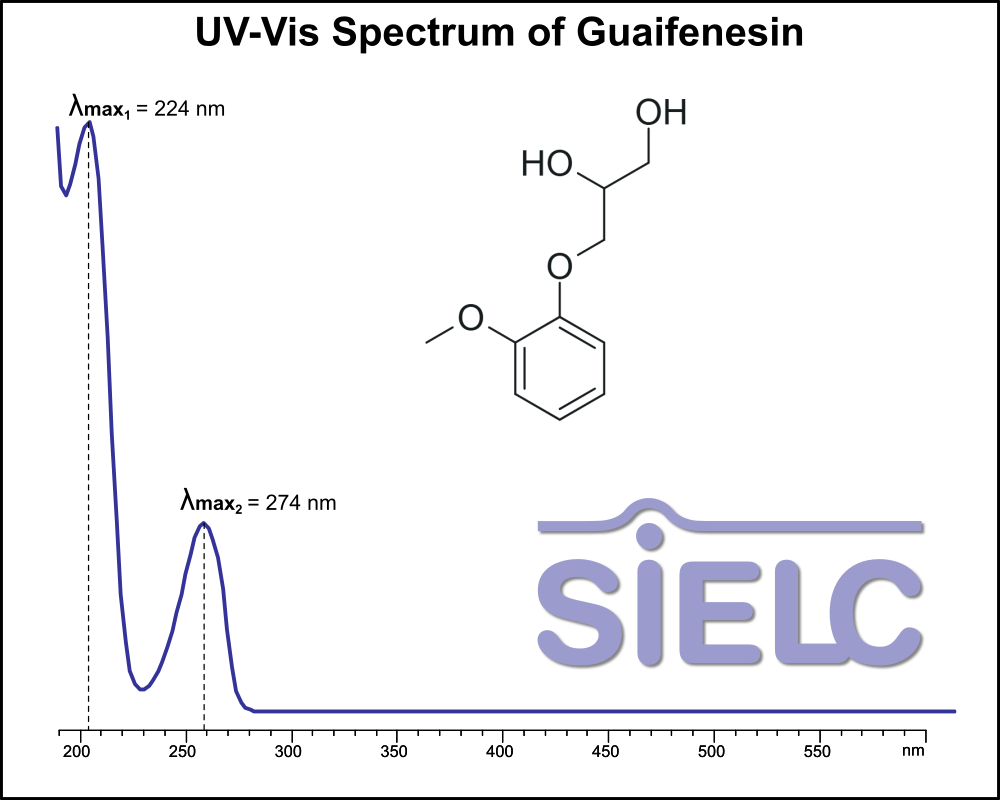
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

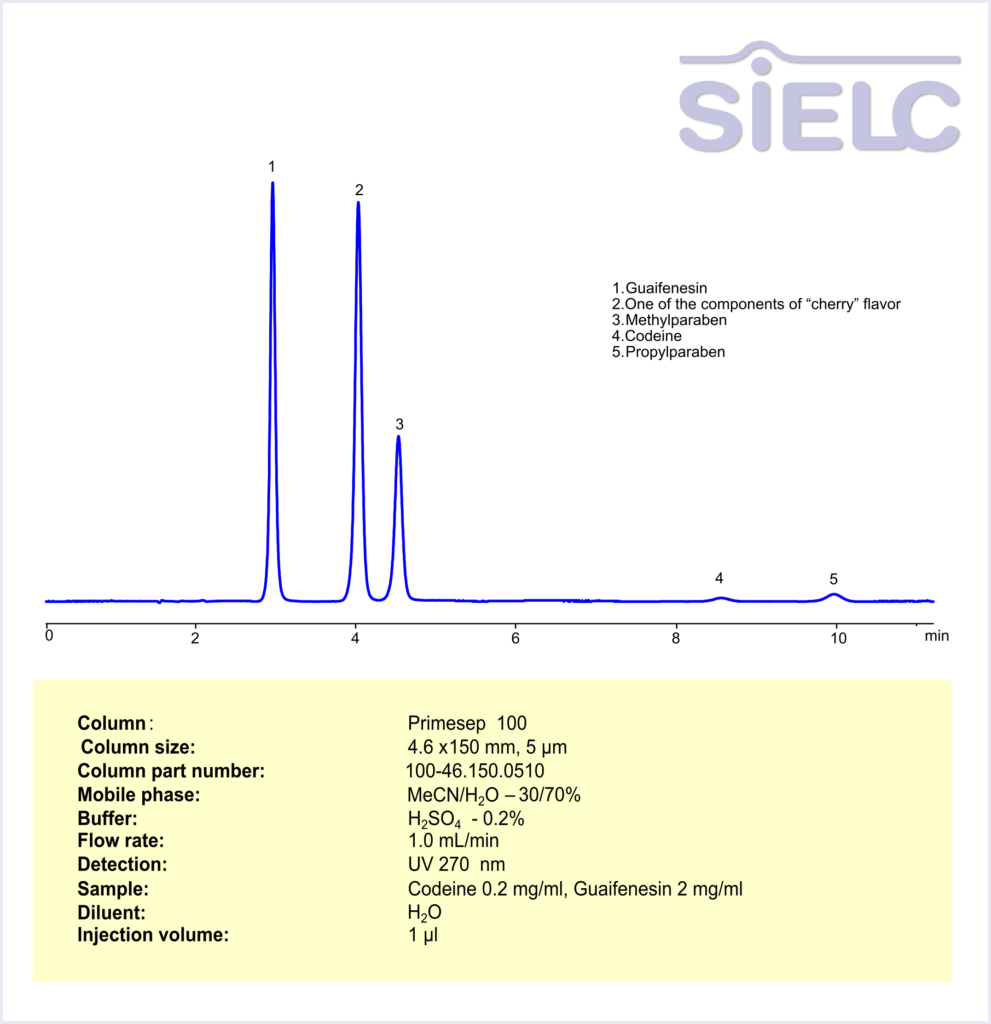
HPLC Method for Analysis of Codeine and Guaifenesin in Cough syrup on Primesep 100 Column
June 16, 2025
HPLC Method for Codeine, Guaifenesin on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Codeine, Guaifenesin
Codeine is a pain reliever and cough suppressant. It belongs to the opioid family (like morphine, but milder). Guaifenesin is an expectorant, which helps loosen mucus in your chest so you can cough it up more easily.
Codeine, Guaifenesin can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid. Detection is performed using UV at 270 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 30% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm |
*LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
| Class of Compounds | Drug |
| Analyzing Compounds | Codeine, Guaifenesin |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Guaifenesin

HPLC Method for Separation of Terbutaline Sulfate, Guaifenesin and Ambroxol on Primesep 200 Column
January 12, 2023
High Performance Liquid Chromatography (HPLC) Method for Separation of Terbutaline Sulfate, Guaifenesin and Ambroxol on Primesep 200 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
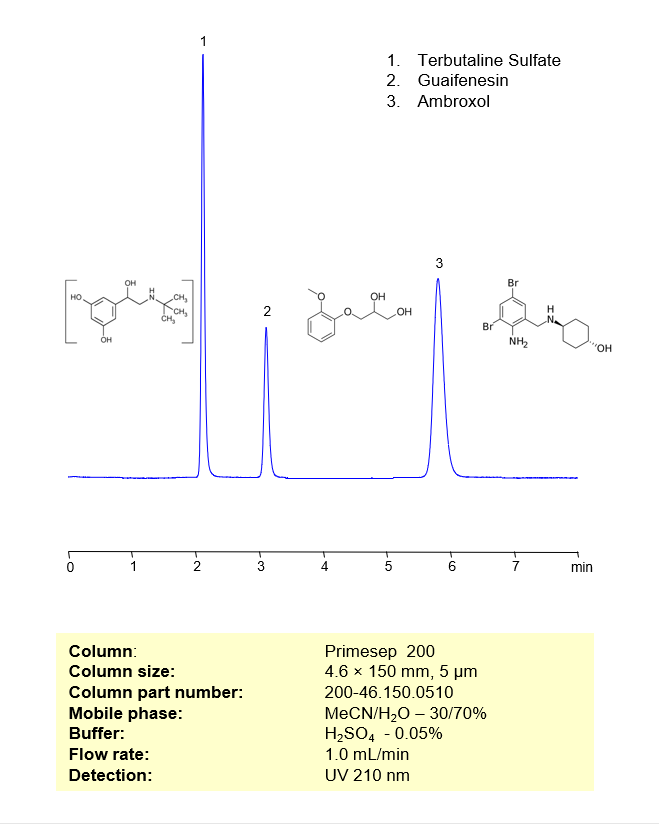
Terbutaline Sulfate is a β2 analyst typically used as a prescription-strength cough suppressant. Guaifenesin, also known as Glyceryl Guaiacolate, is a popular expectorant used to treat chest congestion. Ambroxol is a well-known expectorant that targets and breaks down phlegm. Together, these three medicinal compounds are typically found as the active ingredients in many cough syrups. They can be retained, analyzed, and separated on a Primesep 200 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Phosphoric acid (H3PO4) ionic modifier. This analysis method can be detected in the low UV regime at 210 nm.
High Performance Liquid Chromatography (HPLC) Method for Separation of Terbutaline Sulfate, Guaifenesin and Ambroxol
.
Condition
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H3PO4 – 0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Peak Retention Time | 2.09, 3.28, 5.81 min |
Description
| Class of Compounds | Drugs |
| Analyzing Compounds | Terbutaline Sulfate, Guaifenesin and Ambroxol |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Guaifenesin
Terbutaline

USP Methods for the Analysis of Guaifenesin Using a Legacy L1 Column
June 21, 2012
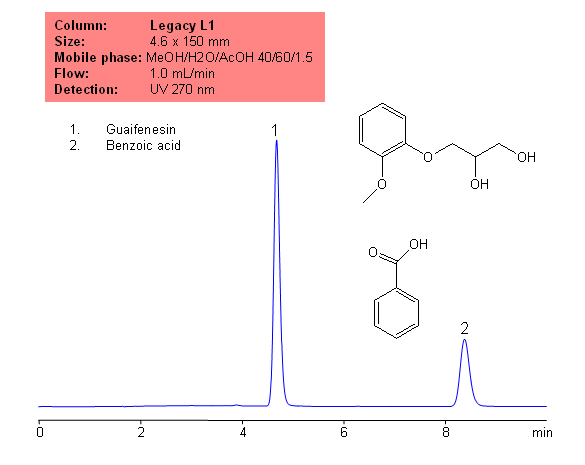
Application Notes: Guaifenesin is common, over the counter expectorant. Guaifenesin contain no less than 98 percent and not more than 102 percent of the labeled amount of guaifenesin calculated on a dried basis, according to the USP methods. the The USP HPLC method for the analysis of guaifenesin was developed on our Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Guaifenesin, benzoic acid
Mobile phase: MeOH/H2O/AcOH 40:60:1.5
Detection technique: UV
Reference: USP 35- NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/H2O/AcOH 40/60/1.5 |
| Buffer | NaH2PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Antibiotics, Hydrophobic, Ionizable, Acid |
| Analyzing Compounds | Guaifenesin, Benzoic acid |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsGuaifenesin

HPLC Separation of Polar Drugs with MS-compatible Method
October 12, 2010
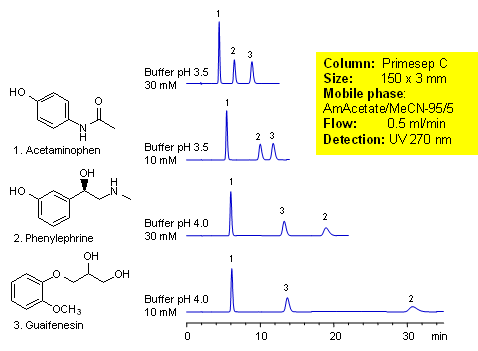
Acetaminophen is an over-the-counter pain and fever reducer, and a major component of cold and flue remedies. Phenylephrine is a decongestant. Guaifenesin is a mucolytic agent used to relieve respiratory difficulties. These three compounds of cough medication were separated by mixed-mode chromatography on a Primesep C HPLC column. Retention and order of elution for phenylephrine can be changed by buffer concentration and buffer pH. Method can be used for analysis of cough and cold composition during the production and in QC/QA environment. Method is compatible with LC/MS and can be used to analyze these components in biological fluids. This generic HPLC method is robust and reproducible.
| Column | Primesep C, 3×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | AmAc |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Acetaminophen, Pseudoephedrine, Guaifenesin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGuaifenesin
Phenylephrine

Effect of pH on Retention of Basic Compounds on Primesep C Columns
January 13, 2010
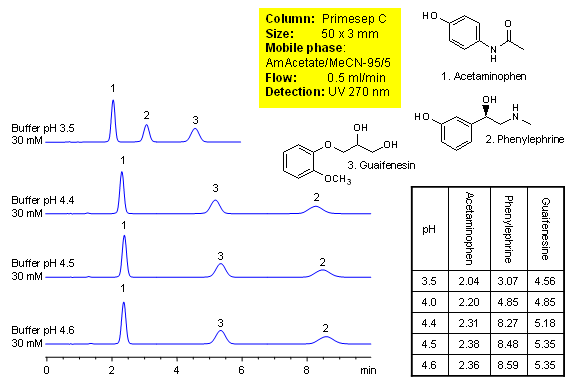
Method for separation of components of cough medication shows how retention for acetaminophen, phenylephrine and guaifenesin is controlled. Order of elution for compounds can be changed based on composition of the mobile phase. The method is compatible with UV, ELSD and LC/MS and can be used for quantitation of drugs in formulation. Primesep C mixed-mode HPLC column is ideal candidate for analysis of cough compositions. Basic compounds are well retained in mixed-mode chromatography without use of ion-pairing reagents. Several companies validated this approach. The method is reproducible and robust and can be used in both production environments and R&D. This HPLC method can be adopted as general approach for analysis of basic compounds in various mixtures.
| Column | Primesep C, 3×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | AmAc |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Acetaminophen, Pseudoephedrine, Guaifenesin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGuaifenesin
Phenylephrine

HPLC Separation of Guaifenesin and Terbutaline on Primesep 200
June 5, 2006
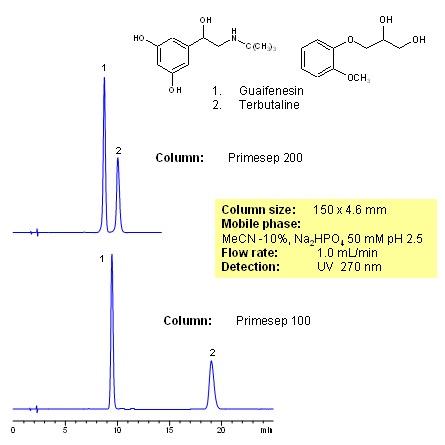
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Terbutaline

HPLC Separation of Guaifenesin and Terbutaline on Primesep 100
June 5, 2006

Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Terbutaline