| CAS Number | 51-41-2 |
|---|---|
| Molecular Formula | C8H11NO3 |
| Molecular Weight | 169.180 |
| InChI Key | SFLSHLFXELFNJZ-QMMMGPOBSA-N |
| LogP | -1.24 |
| Synonyms |
|
Applications:
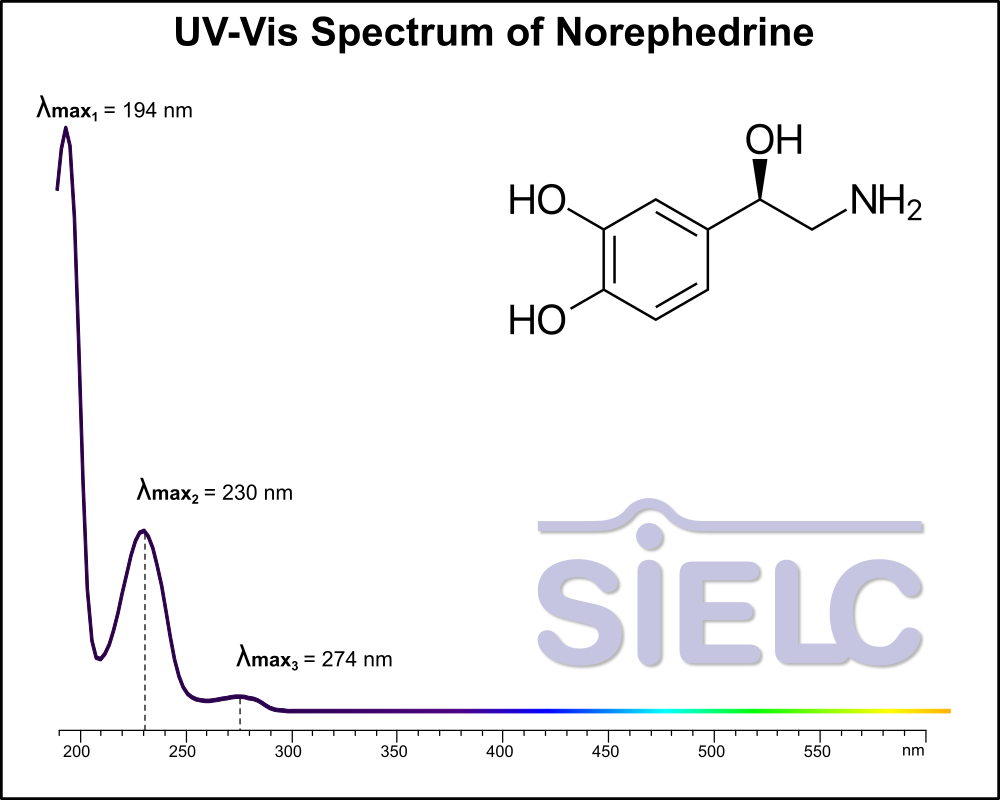
Uv-Vis Spectrum of Norepinephrine
January 23, 2026
If you are looking for optimized HPLC method to analyze Norepinephrine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

UV-Vis Spectrum of Norepinephrine
August 29, 2024
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

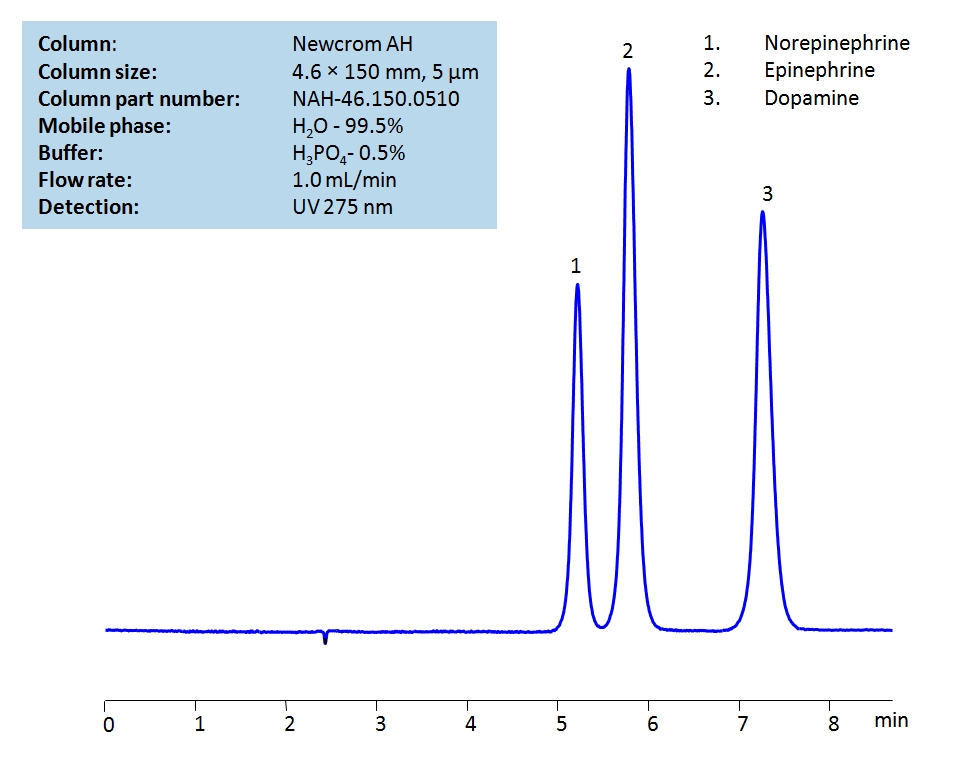
HPLC Separation of Catecholamines on Newcrom AH Column
May 20, 2021
HPLC Method for Dopamine, Epinephrine, Norepinephrine on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Dopamine, Epinephrine, Norepinephrine.
Catecholamines are neurotransmitters that generate the ‘fight-or-flight’ responsein the body. The 3 main catecholamines are epinephrine (also known as adrenaline), norepinephrine, and dopamine, and they are produced by the adrenal glands.
Norepinephrine, also known as noradrenaline and noradrenalin, is a catecholamine with the chemical formula C8H11NO3. It functions as a hormone, neurotransmitter, and neuromodulator responsible for improving alertness, focus, memory, and regulating mood. You can find detailed UV spectra of Norepinephrine and information about its various lambda maxima by visiting the following link.
Epinephrine, also known as adrenaline or adrenalin, is a a hormone and medication with the chemical formula C9H13NO3. As a hormone, it is involved in regulating involuntary operations of nearly all internal organs. As a medication, it is used to treat allergic reaction anaphylaxis and cardiac arrest. When other treatments are not effective, it is occasionally used to treat asthma. You can find detailed UV spectra of Epinephrine and information about its various lambda maxima by visiting the following link.
Dopamine is a neurotransmitter with the chemical formula C8H11NO2. It is released during pleasurable activities, reinforcing those behaviors, and motivating people to continue seeking them out. Dopamine influences the release of other hormones. A deficiency of it can influence movement and lead to conditions like Parkinson’s disease. You can find detailed UV spectra of Dopamine and information about its various lambda maxima by visiting the following link.
Dopamine, Epinephrine, Norepinephrine can be detected in the low UV regime. Using a Newcrom AH mixed-mode column and a mobile phase consisting of almost entirely water with either a phosphoric (H3PO4) acid or ammonium formate (AmFm) buffer, the catecholamines can be retained, separated, and measured. This analysis method can be UV detected at 275 nm with high resolution. The latter mobile phase (utilizing AmFm) is compatible with Mass Spectrometry.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | H2O |
| Buffer | H3PO4 or AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Dopamine, Epinephrine, Norepinephrine |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Epinephrine
Norepinephrine

USP Method for the Analysis of Norepinephrine using the Legacy L1 Column
June 21, 2012
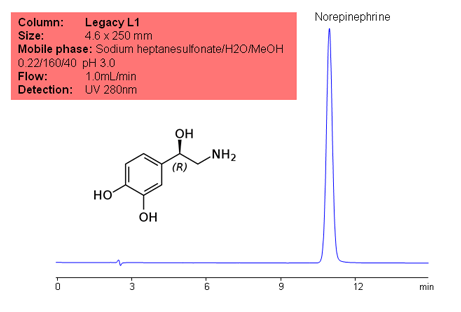
Application Notes: Norepinephrine is a naturally occurring hormone and neurotransmitter. The USP HPLC method for the separation of norepinephrine was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of UPS.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Norepinephrine
Mobile phase: Sodium heptanesulfonate/H2O/MeOH 0.22/160/40 pH 3.0
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | Sodium heptanesulfonate/H2O/MeOH 0.22/160/40 pH 3.0 |
| Buffer | Sodium heptanesulfonate |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 280 nm |
| Class of Compounds |
Drug, Hormone and neurotransmitter, Hydrophilic, Ionizable |
| Analyzing Compounds | Norepinephrine |
Application Column
Legacy L1
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Separation of Catecholamines on Primesep 100 Column with Phosphate Buffers
July 8, 2011
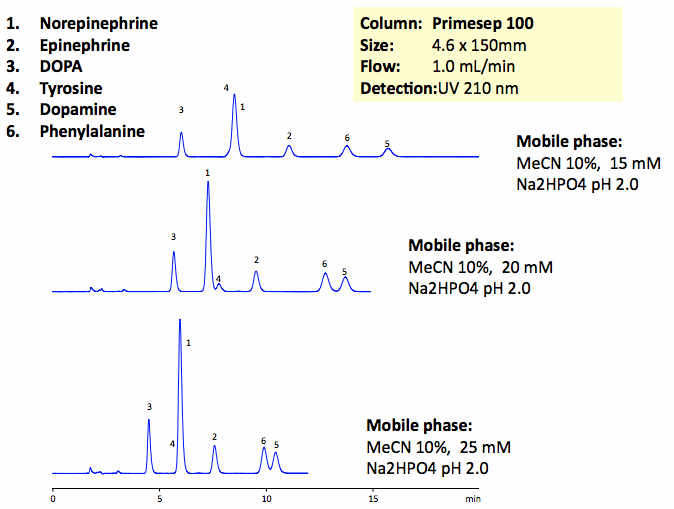
The catecholamine neurotransmitters are amino-acid derivatives of tyrosine. DOPA, tyrosine, phenylalanine, norepinephrine, epinephrine, and dopamine and baseline are resolved on a Primesep 100 column with UV-transparent phosphate buffer. This method can be used for analysis of catecholamines and related impurities in various matrices. Peak order and retention time can be changed by changing the amount of ACN, buffer concentration and buffer pH. Various buffers can be used to accommodate desired detection technique. Primesep 100 is a reversed-phase cation-exchange mixed-mode column that can be used for analysis of polar neutral, polar ionizable, polar zwitter-ionic, hydrophobic neutral, and hydrophobic ionic compounds in the same run. Column can be operated in reverse-phase, cation-exchange, anion-exclusion, HILIC and mixed-modes depending on the mobile phase selection and nature of analytes. Column is compatible with LC/MS and does not require use of ion-pairing reagents.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Na2HPO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Norepinephrine
Phenylalanine
Tyrosine

HPLC Separation of Polar Compounds
January 13, 2010
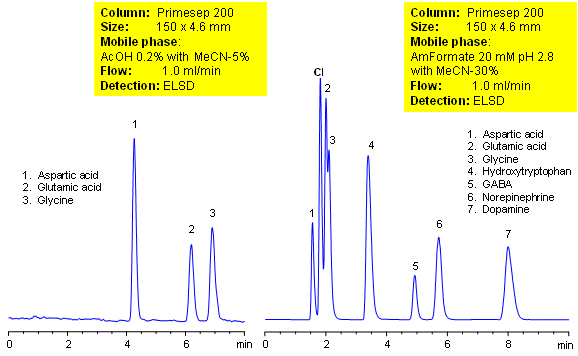
The separation of amino acids, the building blocks of proteins, can be challenging to separate on a reverse-phase column due to their high polarity. Using a mixed-mode HPLC column, allows the separation of amino acids by cation-exchange and ion-exclusion mechanisms as well as hydrophobicity. Fine tuning of separation can be achieved with changes in organic concentration of the mobile phase as well as choice of buffer and pH.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AcOH, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Aspartic acid, Glutaric acid, Glycine, Hydroxytriptophan, GABA, Norepinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAspartic Acid
Dopamine
Glutamic Acid
Norepinephrine
gamma-Aminobutyric Acid (GABA)

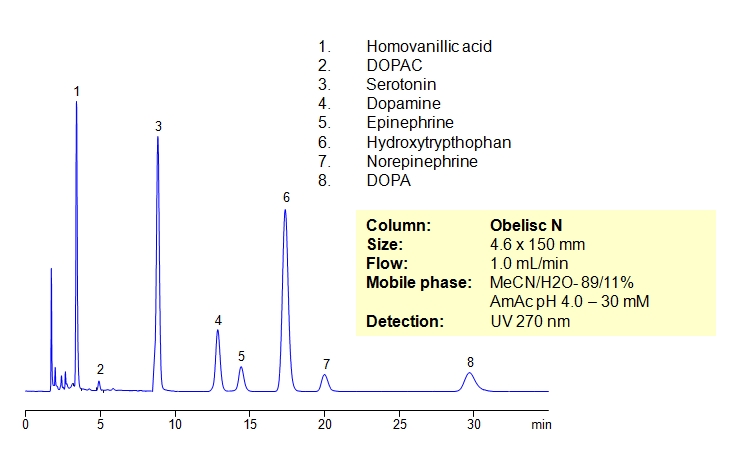
Separation of Serotonin, Dopamine, and Related Compounds
August 22, 2008
Catecholamines are chemical compounds derived from the amino acid tyrosine containing catechol and amine groups. Some of them are biogenic amines. Retention of compounds of the catecholamine pathway is achieved on Obelisc N column. All polar compounds are well retained by combination of HILIC and ion-exchange mechanisms. Obelisc N columns produce very good peak shapes for all analytes. The method is very sensitive to amount of ACN, buffer and buffer pH. The retention time changes with variation of the main parameters. This method can be used for quantitation of biogenic amines and related compounds (homovanillic acid, dihydroxyphenyl acetic acid, serotonin, dopamine, epinephrine, hydroxytryptophan, epinephrine and DOPA) in urine, blood and other biological fluids. Further optimization of this HPLC method can be used during screening and validation. Amines and acids can be analyzed in the same run and retained by a combination of polar organic mode, cation-exchange and anion-exchange modes. Various buffers within specified pH can be employed (ammonium formate, ammonium acetate, sodium phosphate, etc.).
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.0- 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Monocarboxylic acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Homovanillic acid, Dihydroxyphenyl acetic acid, Serotonin, Dopamine, Epinephrine, Hydroxytryptophan, DOPA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsDOPAC (Dihydroxyphenylacetic Acid)
Dopamine
Epinephrine
Homovanillic Acid
Hydroxytryptophan
Norepinephrine
Serotonin

HPLC Separation of Neurotransmitters and Related Drugs
August 22, 2008
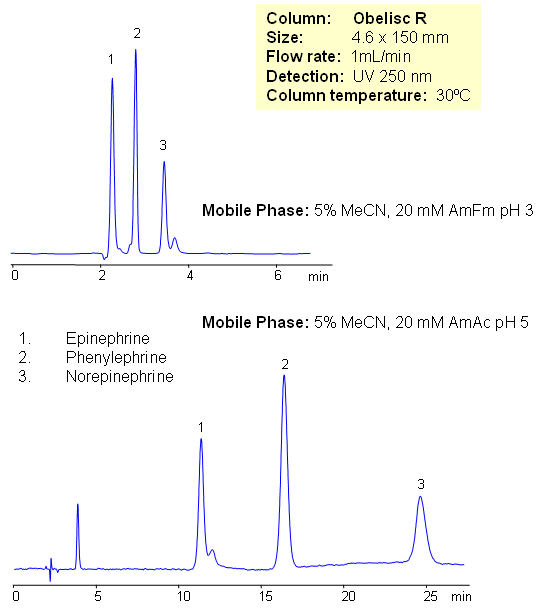
Epinephrine and norepinephrine (adrenaline and noradrenaline) are hormones and neurotransmitters. Epinephrine is synthesized from norepinephrine in a synthetic pathway shared by all catecholamines, including L-dopa, dopamine, norepinephrine, and epinephrine. Phenylephrine is used as a decongestant, available as an oral medicine or as a nasal spray. Phenylephrine is now the most common over-the-counter (OTC) decongestant. All three compounds are used in various drug compositions. Separation of epinephrine and norepinephrine is a challenging task due to polarity and close properties of two compounds. Epinephrine, norepinephrine and phenylephrine are separated in this method on Obelisc R mixed-mode HPLC columns. The method is very sensitive to variation of pH and pH adjustment can be used to achieve desired selectivity and retention time. Other catecholamines can be analyzed using this HPLC method. The method can be used as a stability indicating or a impurity profiling approach to the analysis of neurotransmitters in drug formulation, blood, serum and urine.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Phenylalanine, Norepinephrine, Epinephrine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsNorepinephrine
Phenylephrine

Separation of Epinephrine and Norepinephrine
January 17, 2004
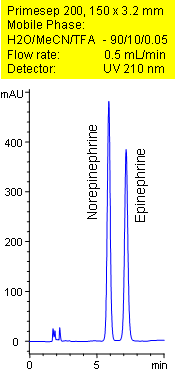
Primesep 200 separates the catecholamines, norepinephrine and epinephrine, less than 8 minutes. Norepinephrine and epinephrine are baseline resolved by a combination of reversed-phase, and ion-exchange mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 200, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Norepinephrine, Epinephrine |
Application Column
Primesep 200
Column Diameter: 3.2 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Norepinephrine