| CAS Number | 120-47-8 |
|---|---|
| Molecular Formula | C9H10O3 |
| Molecular Weight | 166.176 |
| InChI Key | NUVBSKCKDOMJSU-UHFFFAOYSA-N |
| LogP | 2.47 |
| Synonyms |
|
Applications:
HPLC Method for Estimation of Clotrimazole in Health Care Products on Primesep 200 Column
September 11, 2023
HPLC Method for Analysis of Clotrimazole, Benzyl alcohol, Ethylparaben on Primesep 200 by SIELC Technologies
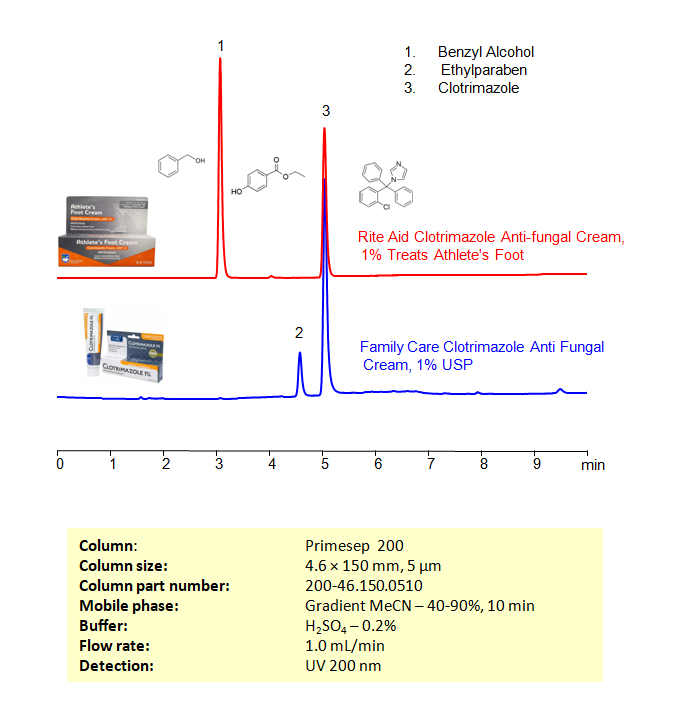
High Performance Liquid Chromatography (HPLC) Method for Analysis of Clotrimazole, Benzyl alcohol, Ethylparaben
Clotrimazole is an antifungal medication widely used in the treatment of fungal infections.
Description:
- Classification: Clotrimazole belongs to the class of medications called imidazole antifungals.
Uses:
- Topical: It’s commonly used to treat skin infections such as athlete’s foot, jock itch, ringworm, and other fungal skin infections (candidiasis).
- Vaginal: As a vaginal cream or tablet, it’s used to treat vaginal yeast infections.
- Otic (Ear): Clotrimazole solution can be used to treat fungal ear infections.
Mechanism of Action:
- Clotrimazole works by inhibiting the synthesis of ergosterol, a vital component of fungal cell membranes. When ergosterol synthesis is inhibited, it leads to structural and functional impairment of the fungal cell membrane, thereby killing the fungus or inhibiting its growth.
Clotrimazole belongs to the class of chemical compounds known as imidazoles. Specifically, it’s an antifungal compound used to treat fungal infections. Imidazoles work by inhibiting the enzyme lanosterol 14α-demethylase, which is necessary for the synthesis of ergosterol, an essential component of fungal cell membranes. When ergosterol synthesis is disrupted, it leads to alterations in the cell membrane’s permeability and, eventually, fungal cell death.
Other antifungal agents in the imidazole class include miconazole, econazole, and ketoconazole, among others. These are commonly used in various formulations to treat a range of fungal infections, including athlete’s foot, ringworm, and yeast infections.
Clotrimazole can be retained, and analyzed on a Primesep 200 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected in the UV 200 nm.
| Column | Primesep 200, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | Gradient MeCN – 40-90%, 10 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Imidazole antifungals |
| Analyzing Compounds | Clotrimazole, Benzyl alcohol, Ethylparaben |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Clotrimazole
Ethylparaben

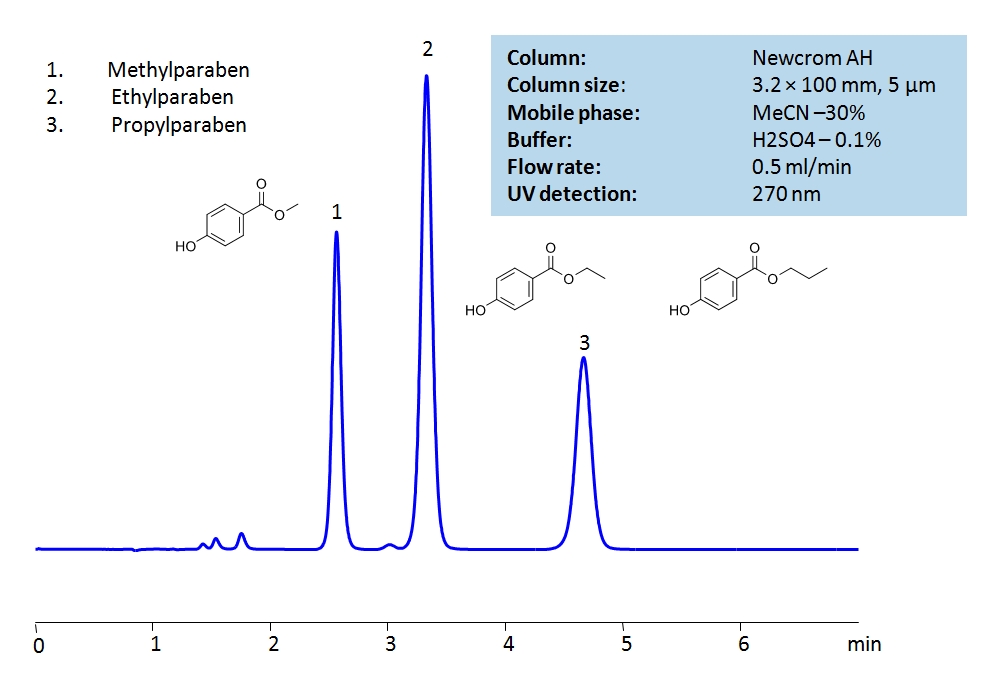
HPLC Analysis of Paraben Preservatives on Newcrom AH Column
October 29, 2020
| Column | Newcrom AH, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Preservatives, Hydrophobic |
| Analyzing Compounds | Methyl Paraben, Ethyl Paraben, Propyl Paraben |
Application Column
Newcrom AH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsMethylparaben
Methylparaben sodium
Parabens
Propylparaben
Propylparaben sodium

Generic Screening Method for Complex Mixtures on Primesep 200
October 15, 2015
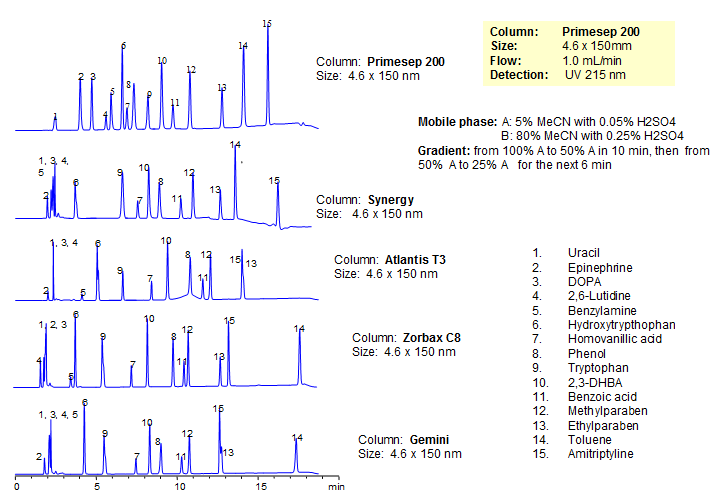
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Analysis of Active Drug and Amino Acids in a Formulation
October 14, 2010
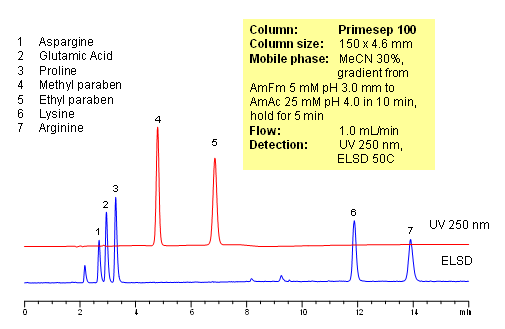
Polar amino acids are very often used as components of vitamin and supplement composition. Analysis of such complex composition is a challenging task. In this application, 5 amino acids (asparagine, glutamic acid, proline and arginine) and two preservatives (methyl paraben and propyl paraben) are separated on a Primesep 100 reversed-phase cation-exchange column with LC/MS compatible mobile phase. Method does not require ion-pairing reagent in the mobile phase. Compounds are monitored by ELSD and UV. Method is validated for quantitation of underivatized amino acids in complex mixtures. The method is simple and robust and can be used for analysis of various vitamin formulations.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD 50C, UV 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Glutamic acid, Aspargine, Proline, Lysine, Arginine, Methyl paraben, Propyl paraben |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAsparagine
Ethylparaben
Glutamic Acid
Lysine
Methylparaben
Proline
UV Detection