| CAS Number | 94-13-3 |
|---|---|
| Molecular Formula | C10H12O3 |
| Molecular Weight | 180.204 |
| InChI Key | QELSKZZBTMNZEB-UHFFFAOYSA-N |
| LogP | 3.04 |
| Synonyms |
|
Applications:
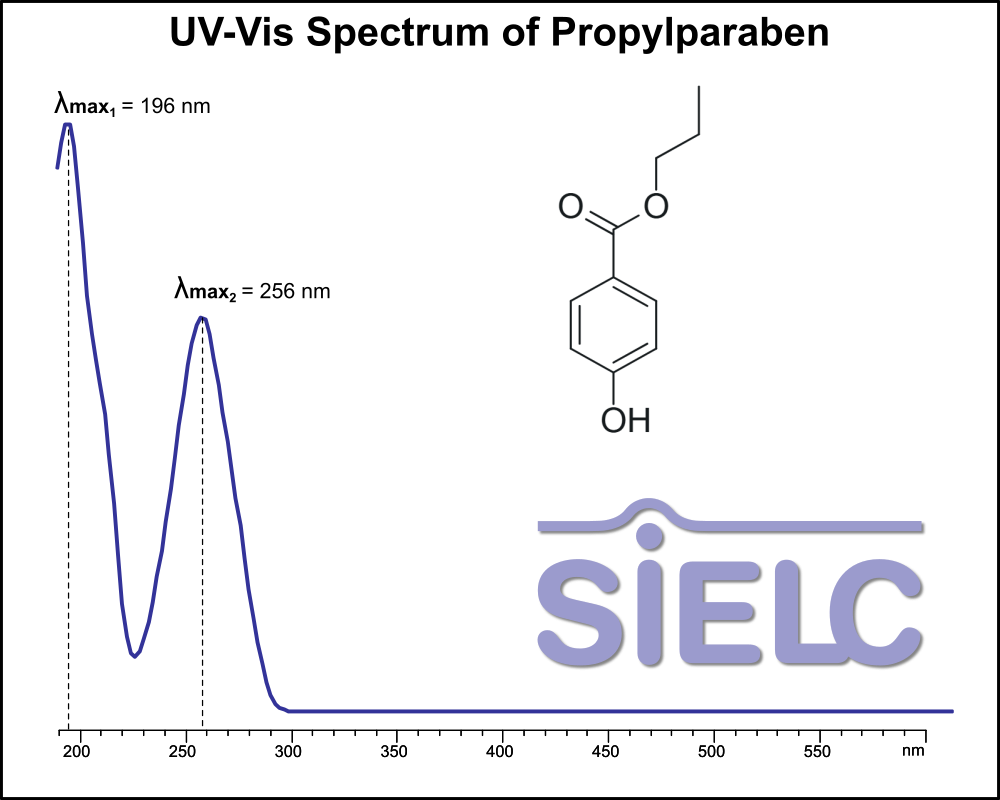
UV-Vis Spectrum of Propylparaben
July 11, 2025
If you are looking for optimized HPLC method to analyze Propylparaben check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

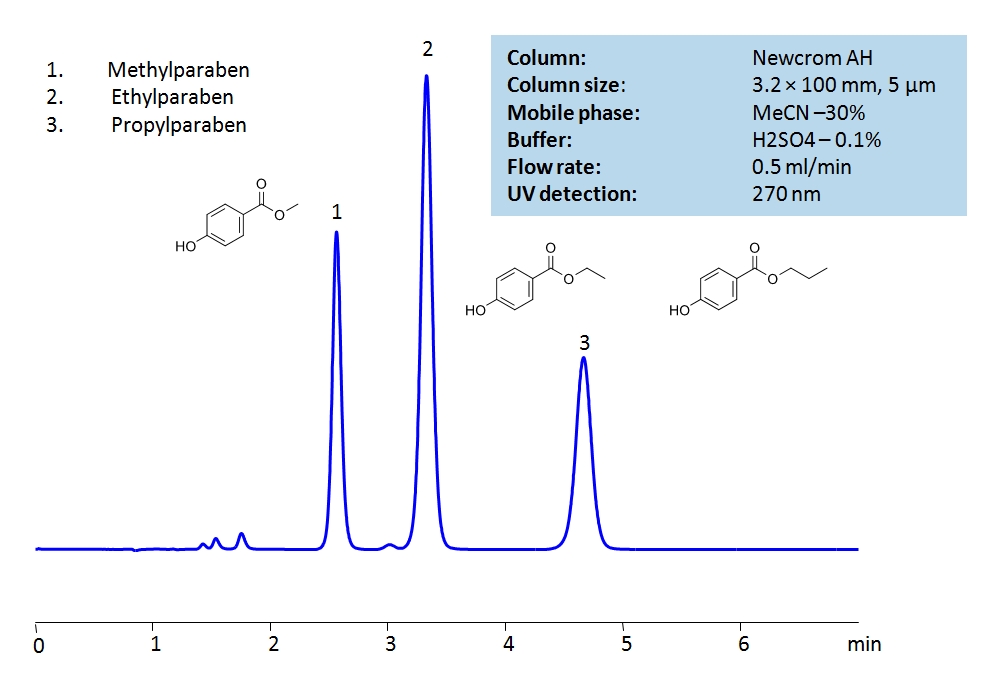
HPLC Analysis of Paraben Preservatives on Newcrom AH Column
October 29, 2020
HPLC Method for Ethylparaben, Methylparaben sodium, Propylparaben sodium, Methylparaben, Parabens, Propylparaben on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ethylparaben, Methylparaben sodium, Propylparaben sodium, Methylparaben, Parabens, Propylparaben.
Methylparaben, also known as Methyl 4-hydroxybenzoate, is a paraben with the chemical formula C8H8O3. It is used as a preservative in food, cosmetics, and pharmaceuticals as it is said to have antimicrobial and antifungal properties. It is considered safe for use in low concentrations, but it may cause irritation or contact dermatitis in rare cases and for those who are allergic.
Ethylparaben is an ethyl ester of p-hydroxybenzoic acid with the chemical formula C9H10O3. It is used as a preservative in food, cosmetics, and pharmaceuticals due to it’s antimicrobial and antifungal properties. There are ongoing debates in regards to it’s safety as it relates to human consumption,
Propylparaben is a n-propyl ester with the chemical formula C10H12O3. It is used as a preservative in food, cosmetics, and personal care products due to it’s antimicrobial properties. Studies show that it absorbs through the skin and remains in the body, leading to the European Union banning it. California had banned it for use in food, but not in other uses.
You can find detailed UV spectra of Methylparaben and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Ethylparaben and information about its various lambda maxima by visiting the following link.
You can find detailed UV spectra of Propylparaben and information about its various lambda maxima by visiting the following link.
Ethylparaben, Methylparaben sodium, Propylparaben sodium, Methylparaben, Parabens, Propylparaben can be retained and analyzed using the Newcrom AH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Newcrom AH, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Preservatives, Hydrophobic |
| Analyzing Compounds | Ethylparaben, Methylparaben sodium, Propylparaben sodium, Methylparaben, Parabens, Propylparaben |
Application Column
Newcrom AH
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Methylparaben
Methylparaben sodium
Parabens
Propylparaben
Propylparaben sodium

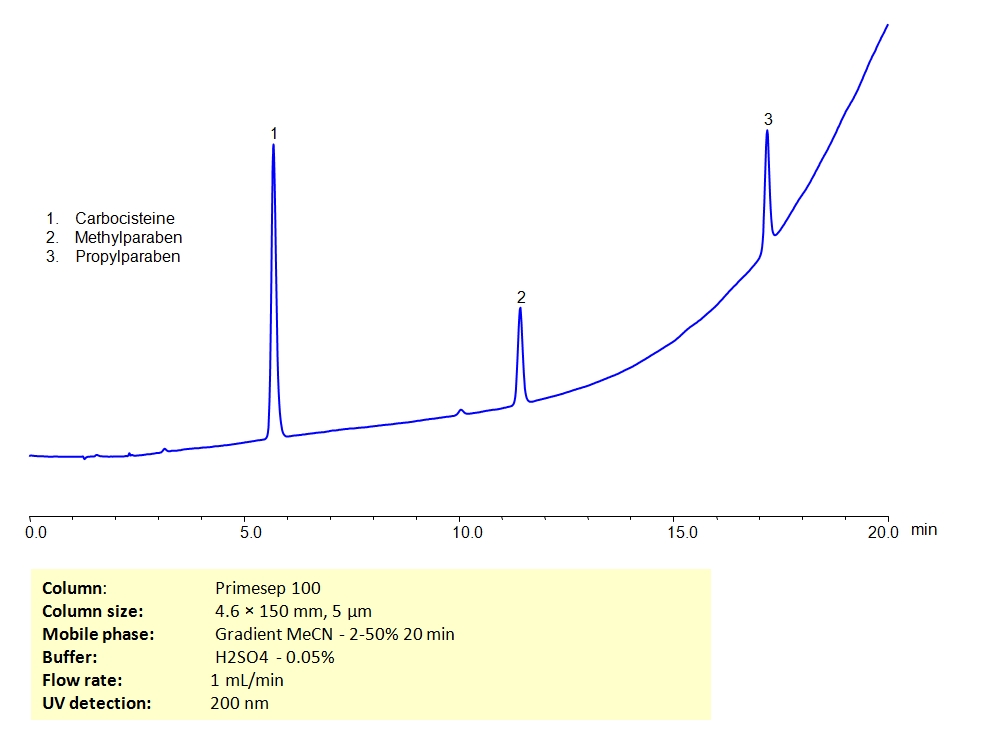
HPLC Separation of Mixture of Carbocisteine, Methylparaben & Propylparaben on Primesep 100 Column
July 31, 2019
HPLC Method for Carbocisteine, Methylparaben sodium, Propylparaben sodium, Propylparaben, Methylparaben on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Carbocisteine, Methylparaben sodium, Propylparaben sodium, Propylparaben, Methylparaben.
Carbocysteine is a medication that reduces the viscosity of mucus making it easier to cough up. Methylparaben and propylparaben are used as preservatives in cosmetics, pharmaceuticals and food. They can be separated in HPLC on a Primesep 100 mixed-mode column by both hydrophobic and ionic properties present on the stationary phase. The analytical method uses acetonitrile (ACN) gradient and water with sulfuric acid (H2SO4) as buffer and UV detected at 200nm.
| Column | Primesep 100 |
| Mobile Phase | Gradient MeCN – 2-50%, 20 min |
| Buffer | H2SO4 – 0.05 % |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Carboxylic acid, Carbocyclic, Benzoate, Hydroxy Acid, Amino Acid, Hydroxybenzoate, Phenol |
| Analyzing Compounds | Carbocisteine, Methylparaben sodium, Propylparaben sodium, Propylparaben, Methylparaben |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Methylparaben
Methylparaben sodium
Propylparaben
Propylparaben sodium

HPLC Analysis of Propylparaben
July 31, 2015
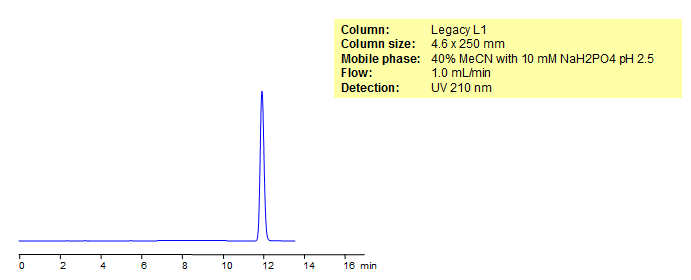
Propyl 4-hydroxybenzoate or propylparaben is a very useful compound in cosmetics and often used as a preservative in lotions and creams. The reverse-phase column Legacy L1 was used to retain propyl 4-hydroxybenzoate. Comparisons to Phenomenex columns are available upon request.
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 430% |
| Buffer | NaH2PO4 pH 2.5 – 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Preservative, Hydrophobic, Ionizable |
| Analyzing Compounds | Phenoxymethylpenicillin |
&
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsUV Detection

Analysis of Codeine-based Drug Composition. Effect of Buffer Concentration and Buffer PH
July 3, 2013
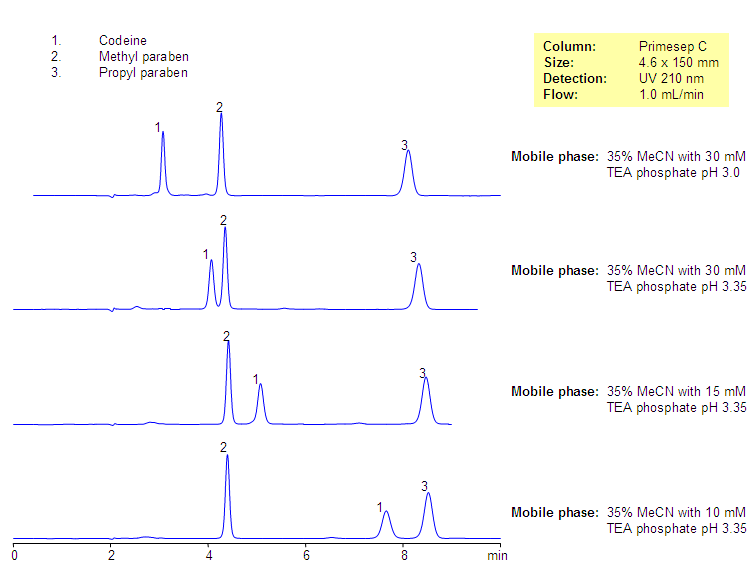
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | TEAPh Ph 3.5 – 15 Mm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Preservatives, Base, Hydrophobic, Ionizable |
| Analyzing Compounds | Codeine, Methyl Paraben, Propyl Paraben |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylparaben
Propylparaben

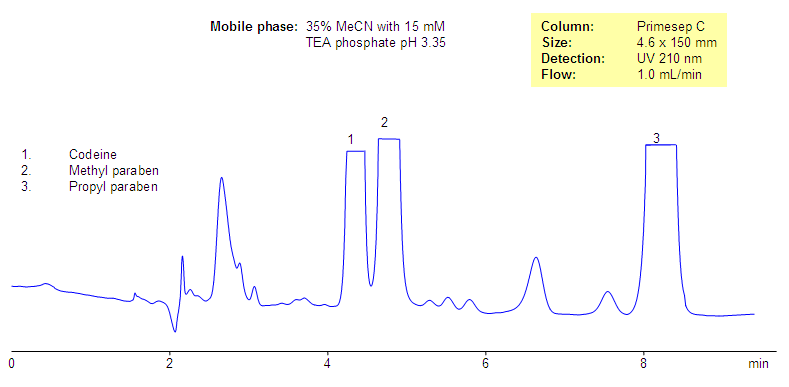
Analysis of Codeine and Related Impurities in Drug Composition
July 3, 2013
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | TEAPh Ph 3.5 – 15 Mm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Preservatives, Base, Hydrophobic, Ionizable |
| Analyzing Compounds | Codeine, Methyl Paraben, Propyl Paraben |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylparaben
Propylparaben

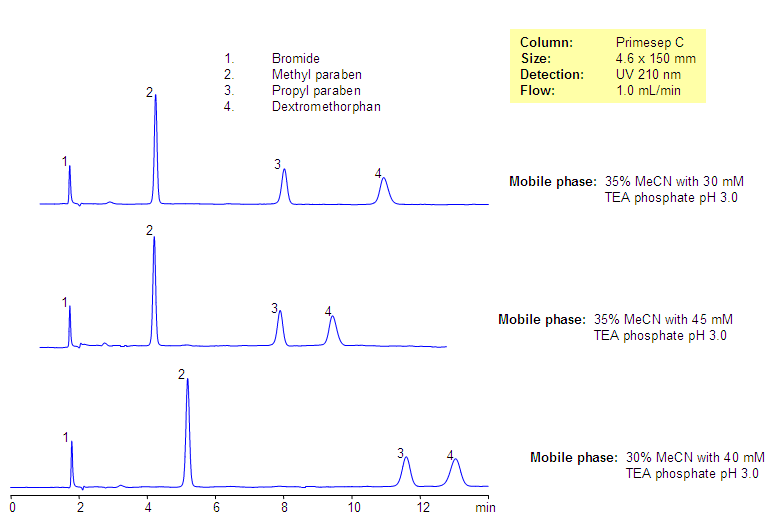
Analysis of Dextromethorphan-Based Composition
July 3, 2013
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TEAPh |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Preservatives, Base, Hydrophobic, Ionizable |
| Analyzing Compounds | Dextromethorphan, Bromide, Methyl Paraben, Propyl Paraben |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDextromethorphan
Methylparaben
Propylparaben

Analysis of Dextromethorphan-Based Drug composition. Effect on buffer pH
July 3, 2013
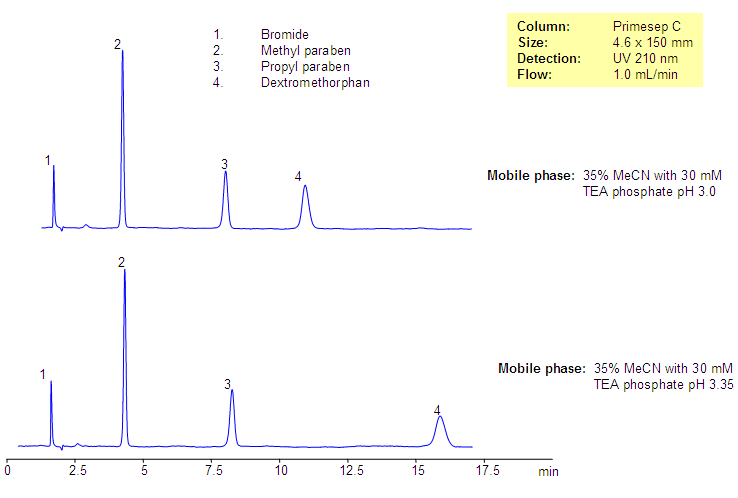
Dextromethorphan is one of the common cough suppressants used in many drug composition. It is in tablets and syrups as an antitussive drug. Composition often has preservatives like parabens. Dextromethorphan is a hydrophobic, basic drug which is used as a bromide salt in drug compositions. Dextromethorphan and two parabens (methyl paraben and propyl paraben) were separated on Primesep C reversed-phase cation-exchange column. Several impurities were observed and are well separated from the main components of the drug composition. Method can be used for various formulations in QC and production environment.
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | TEAPh |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Preservatives, Base, Hydrophobic, Ionizable |
| Analyzing Compounds | Dextromethorphan, Bromide, Methyl Paraben, Propyl Paraben |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDextromethorphan
Methylparaben
Propylparaben

Analysis of Codeine-Based Drug Composition. Effect of Buffer Concentration and Buffer pH
July 2, 2013
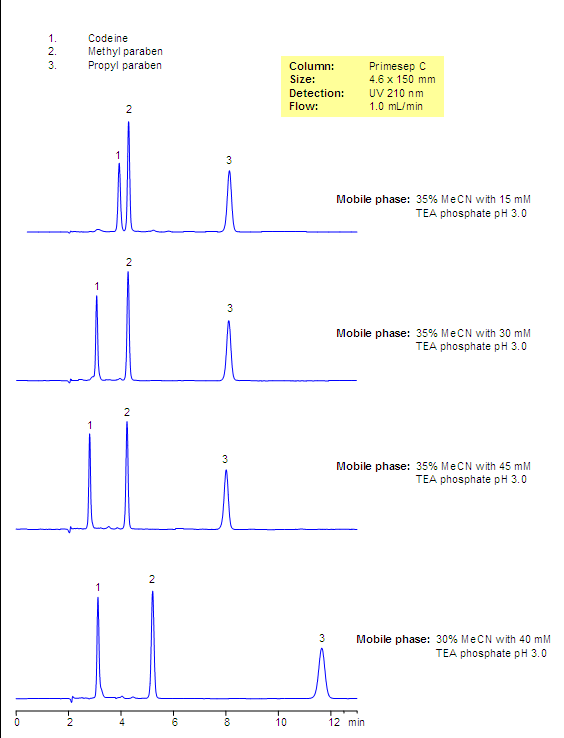
Codeine and two parabens are separated by mixed-mode chromatography. Codeine is retained by reversed-phase and cation-exchange mechanisms and parabens are retained by reversed-phase mechanism. The retention time of codeine can be adjusted by changing the amount of acetonitrile, buffer concentration, and buffer pH. The mobile phase for this column and its separation are fully compatible with UV, ELSD, LC/MS and prep chromatography, various organic and inorganic acids, and corresponding buffers can be used.

Codeine is a hydrophobic basic drug which is used in many drug compositions as an analgetic, antitussive, anxiolytic, and sedative agent. Codeine is widely used as a moderate pain and cough reliever. It is usually part of complex composition and comes in the form of a tablet or syrup. Several preservatives are used in most of the drug composition and include parabens and benzoates. The mixture of codeine, methyl and propyl parabens was separated on Primesep C mixed-mode reversed-phase cation-exchange column. Codeine is retained by reversed-phase and cation-exchange mechanisms and parabens are retained by reversed-phase mechanism. No ion-pairing reagent is required since Primesep C mixed-mode stationary phase has an ion-pairing reagent attached to the surface.
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylparaben
Propylparaben

HPLC Separation of Methyl Paraben, Benzonitrile, Propyl Paraben, and Toluene on Mixed-Mode and Reverse Phase Columns
October 4, 2010
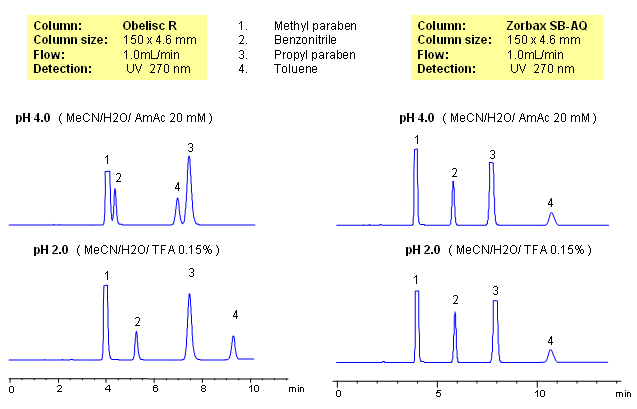
Parabens are common preservatives in pharmaceutical and cosmetic industries. They are esters of p-hydroxybenzoic acid. Method for separation of methyl paraben, propyl paraben, benzonitrile and toluene was developed on a Obelisc R column. All four compounds are neutral and are retained by reverse-phase mechanism. In case of reversed-phase stationary phase, no effect of pH is observed. Retention time for all four compounds changes on an Obelisc R column when pH is changed. pH of the mobile phase affects ionization state of stationary phase. Obelisc R column has C12 carbon chain and carboxylic acid with pKa of 4. At lower pH (pH 2, TFA), carboxylic acid of stationary phase is not ionized and thus adds hydrophobicity to stationary phase. Obelisc R column can be used for analysis of basic, acidic and neutral compounds with suitable detection techniques – UV, ELSD, CAD, LC/MS.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc, TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Preservatives, Neutral |
| Analyzing Compounds | Methylparaben, Benzonitrile, Propyl paraben, Toluene |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsMethylparaben
Propylparaben
Toluene

HPLC Separation of Polar and Hydrophobic Drugs on Obelisc R
March 3, 2007
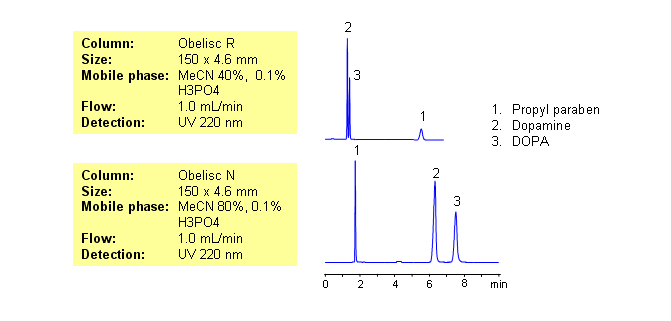
| Column | Obelisc R, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 220 nm |
| Class of Compounds |
Drug, Acid, Preservative, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Propyl paraben, Dopamine, DOPA |
Application Column
Obelisc R
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Dopamine
Propylparaben

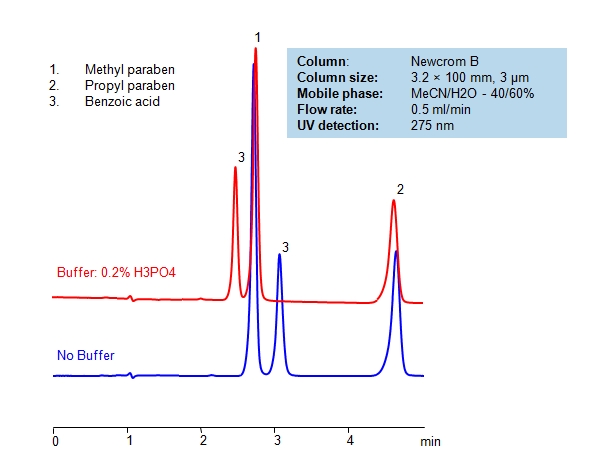
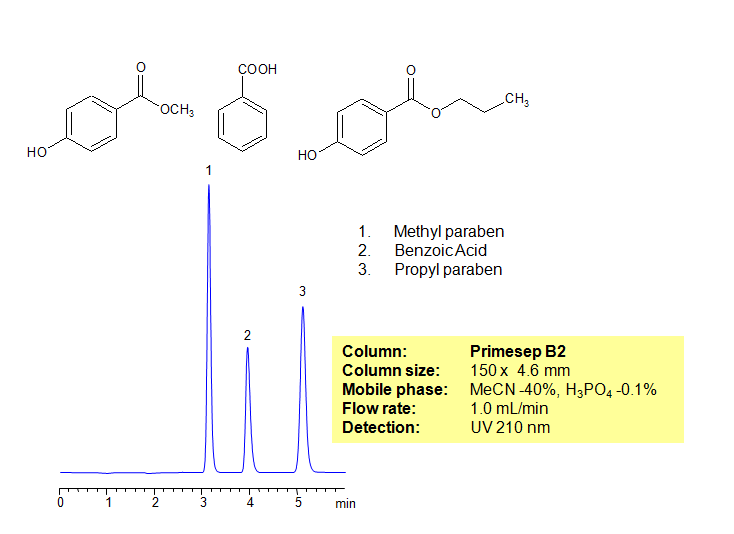
HPLC Separation of Parabens and Benzoic Acid
June 5, 2006
| Column | Newcrom B, 3.2×100 mm, 3 µm, 100A |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | |
| Flow Rate | 0.5 ml/min |
| Detection | UV 275nm |
Parabens possess antibacterial and antifungal properties and are therefore widely used in pharmaceutical and cosmetic industries as preservatives in products. Parabens and benzoic acid can be baseline separated in a short time frame using Primesep B2 reverse-phase HPLC column with a simple mobile phase of water, acetonitrile (ACN, MeCN) and phosphoric acid of 0.1% as buffer. UV detection at 210nm.
| Column | Primesep B2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Preservative |
| Analyzing Compounds | Methyl paraben, Benzoic Acid, Propyl paraben |
Application Column
Newcrom B
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrimesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylparaben
Parabens
Propylparaben

HPLC Separation of Polar and Hydrophobic Drugs on Obelisc N Cloumn
June 11, 2004

| Column | Obelisc N, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 220 nm |
| Class of Compounds |
Drug, Acid, Preservative, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Propyl paraben, Dopamine, DOPA |
Application Column
Obelisc N
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Dopamine
Propylparaben