| CAS Number | 69-89-6 |
|---|---|
| Molecular Formula | C5H4N4O2 |
| Molecular Weight | 152.114 |
| InChI Key | LRFVTYWOQMYALW-UHFFFAOYSA-N |
| LogP | -0.730 |
| Synonyms |
|
Applications:
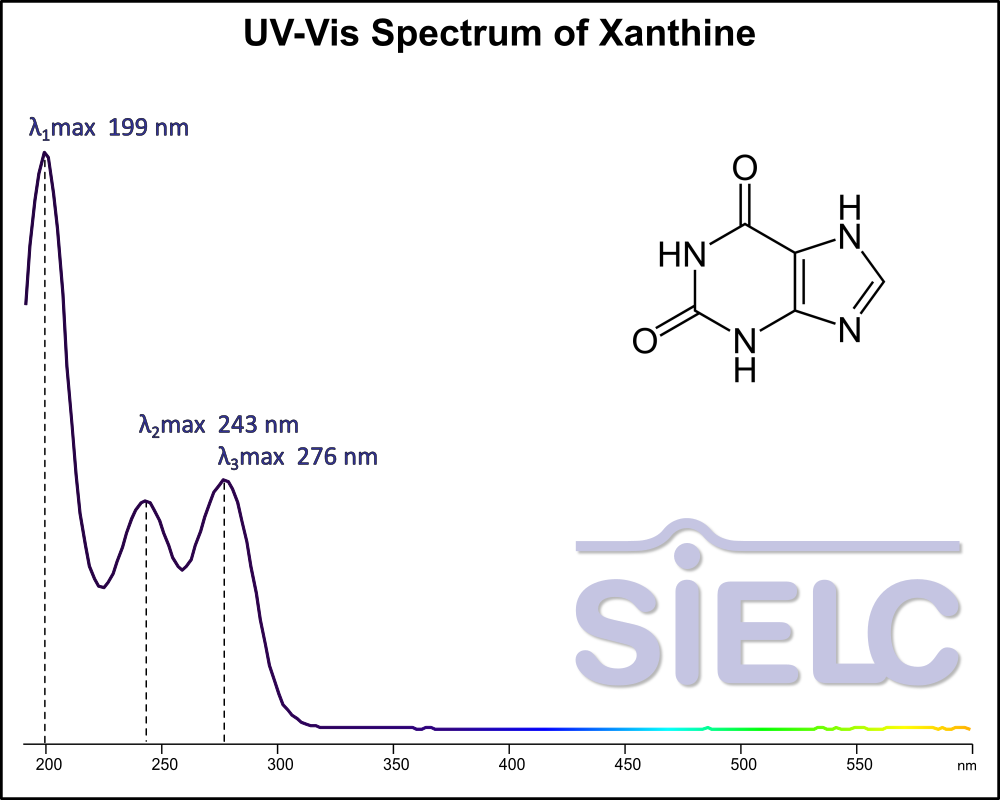
Uv-Vis Spectrum of Xanthine
January 30, 2026
If you are looking for optimized HPLC method to analyze Xanthine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis mixture of Xanthines and Uric Acid BIST B+ by SIELC Technologies
November 16, 2022
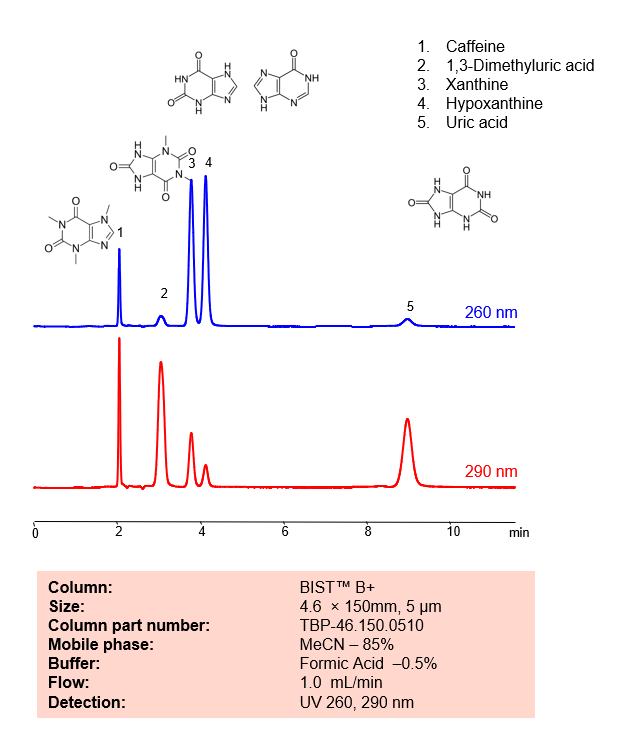
HPLC Method for Analysis mixture of Caffeine, 1,3-Dimethyluric acid, Xanthine, Hypoxanthine, Uric acid by SIELC Technologies.
Xanthines and uric acid are related compounds in the body and both are involved in the metabolism of purines.
Xanthines are a group of alkaloids that are widely distributed in plants, and also occur in the tissues and fluids of animals. They are known to stimulate the central nervous system and cardiac muscle, and also have diuretic effects.
In the body, xanthines are intermediates in the degradation of adenosine monophosphate to uric acid. This metabolic pathway starts with adenosine monophosphate (AMP), which is deaminated to form inosine monophosphate (IMP). IMP is then converted into a xanthine known as hypoxanthine. Hypoxanthine is then oxidized to xanthine, and finally, xanthine is further oxidized to uric acid. Both of the oxidation steps are catalyzed by the enzyme xanthine oxidase.
Caffeine is a natural stimulant and methylxanthine alkaloid. with the molecular formula C6H10N4O2. Caffeine can be found in a variety of plants, including tea, coffea, cocoa, kola nuts, and guarana. Ingestion of it can increase alertness and cognitive function. It can also cause worsening anxiety, heart palpitations, and headaches You can find detailed UV spectra of caffeine and information about its various lambda maxima by visiting the following link.
1,3-Dimethyluric acid is an oxopurine with the chemical formula C7H8N4O3. It is a is a metabolite of caffeine and theophylline with antioxidant properties. It is used as a biomarker in urine for activity of the CYP1A2 enzyme.
Xanthine is a purine base with the chemical formula C5H4N4O2. It is a product on the pathway of purine degradation. Numerous stimulants are derived from xanthine.
Hypoxanthine is a naturally occurring purine derivative with the chemical formula C5H4N4O. It is a reaction intermediate in the metabolism of adenosine as well as a metabolite found in Escherichia coli.
Uric acid is a waste product that’s produced when the body breaks down purines, substances found in foods and drinks like liver, anchovies, mackerel, dried beans, peas, and beer. It is normally excreted from the body in urine. However, if the body produces too much uric acid or doesn’t excrete enough of it, it can build up in the blood and potentially lead to health problems such as gout and kidney stones
Caffeine, 1,3-Dimethyluric acid, Xanthine, Hypoxanthine, Uric acid can be retained, analyzed, and separated using an isocratic analytical method on a BIST B+ column. The simple mobile phase for this method comprises water, acetonitrile (MeCN), and formic acid as an ionic modifier. The analytical method can be monitored with UV detection at 260 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
Condition
| Column | BIST B+, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 85% |
| Buffer | FA – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260, 290 nm |
| Peak Retention Time | 2.01, 3.02, 4.2, 9.09 min |
Description
| Class of Compounds | Acid, Xanthines |
| Analyzing Compounds | Caffeine, 1,3-Dimethyluric acid, Xanthine, Hypoxanthine, Uric acid |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Caffeine
Hypoxanthine
Uric acid
Xanthine

HPLC Separation of Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine
June 15, 2012
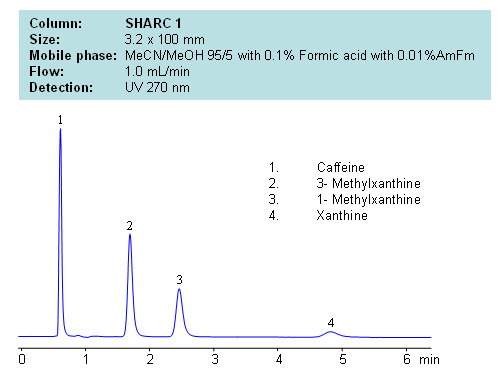
Application Notes: Xanthines are polar neutral compounds which are hard to retain and separate by traditional reversed-phase chromatography. However a hydrogen bonding method makes separation possible due to an observable correlation between the number of hydrogens available for interaction and retention time. Molecules with no hydrogens available for interactions retain less, and compound with multiple hydrogen donors retain the most. Retention time can be controlled by changing ratio of ACN:MeOH. Other protic and aprotic solvents can be used to control retention time and selectivity of separation.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Caffeine, 3-methylxanthine, 1-methylxanthine, and xanthine
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Methylxanthine
Caffeine
Xanthine

HILIC Retention of Polar Compounds on Primesep S
August 22, 2010
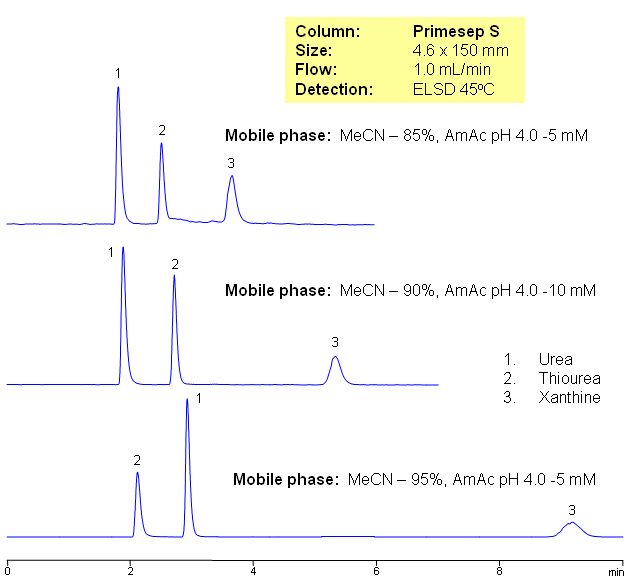
Three neutral polar compounds (urea, thiourea, and xanthine) are separated on a Primesep S HILIC cation-exchange column with LC/MS compatible mobile phase. The retention time is controlled by the amount of acetonitrile in the mobile phase.
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsUrea
Xanthine