| CAS Number | 56-89-3 |
|---|---|
| Molecular Formula | C6H12N2O4S2 |
| Molecular Weight | 240.290 |
| InChI Key | LEVWYRKDKASIDU-IMJSIDKUSA-N |
| LogP | -5.08 |
| Synonyms |
|
Applications:
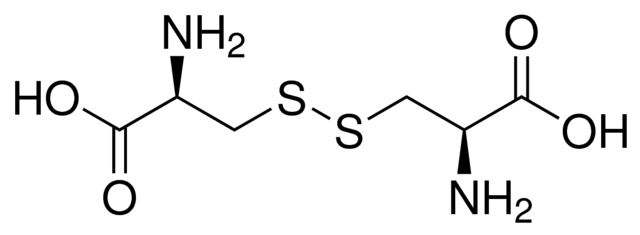
HPLC Method for Analysis of Cystine on Primesep 100 Column
August 7, 2025
HPLC Method for Cystine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cystine
Cystine is an organic compound with the molecular formula C6H12N2O4S2.
Properties:
Appearance: Typically appears yellowish with a waxy, compact, and partially opaque texture.
Molecular weight: ~240.3 g/mol
Solubility: Soluble in water with HCL or NaOH.
Uses: Plays a crucial role in various biological processes due to its ability to stabilize protein structures and its role as a precursor to the antioxidant glutathione.
Cystine can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid. Detection is performed using UV at 200 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds | Disulfide Amino Acid |
| Analyzing Compounds | Cystine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

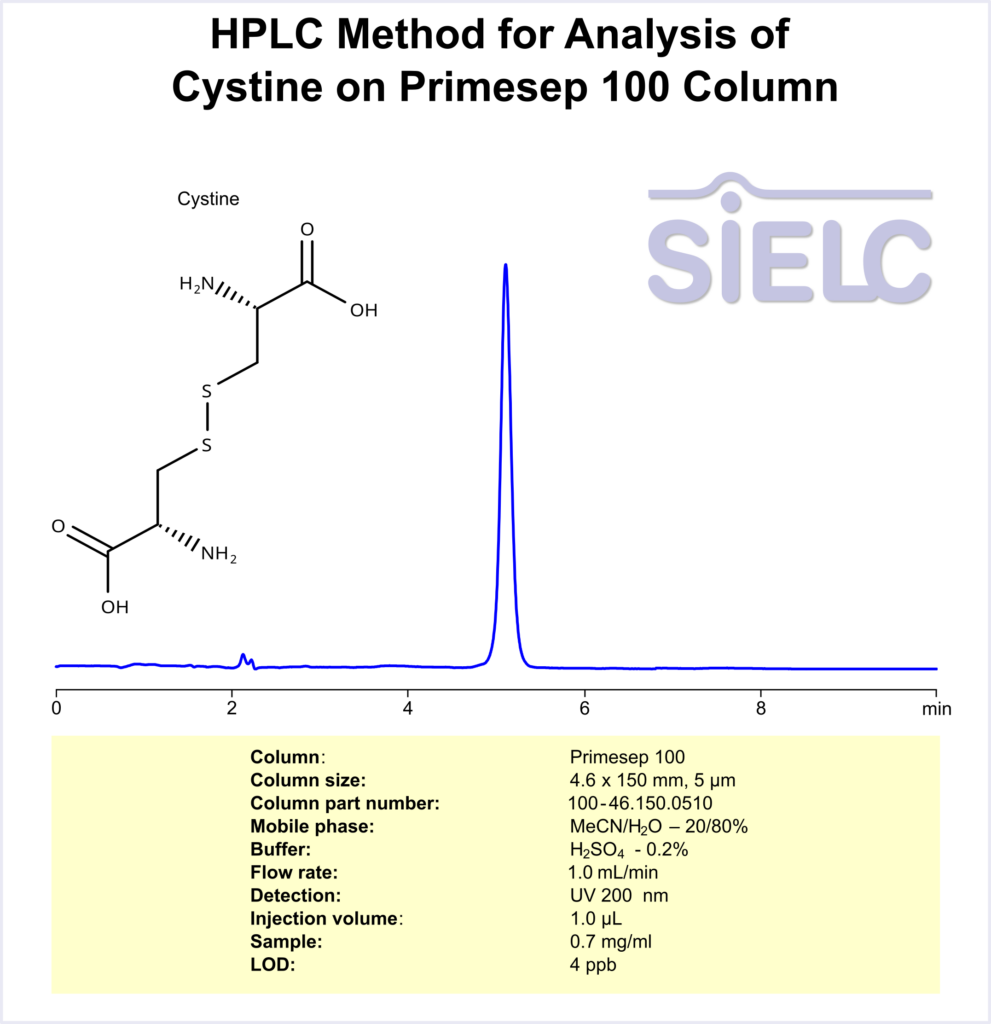
UV-Vis Spectrum of Cystine
August 5, 2025
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Separation of Sulfur-containing Biomolecules on Primesep 100 Column
June 13, 2023
HPLC Method for Separation of Cysteine, Glutathione, reduced, Cystine, Cysteine-glutathione disulfide, Glutathione oxidized on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
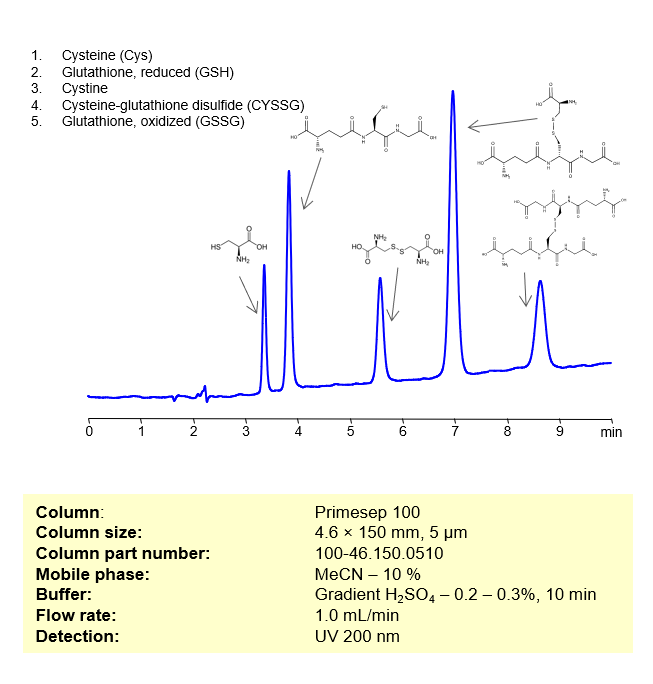
These compounds are all involved in redox reactions and cellular defenses against oxidative stress in biological systems. Here’s a bit more about each of them:
- Cysteine (Cys): This is a sulfur-containing amino acid that’s used in the biosynthesis of proteins. Its thiol side chain often participates in enzymatic reactions, and contributes to the stability of proteins by forming disulfide bonds.
- Glutathione, reduced (GSH): A tripeptide (small protein) consisting of the amino acids glutamic acid, cysteine, and glycine. GSH serves as an antioxidant, helping to prevent damage to cellular components caused by reactive oxygen species such as free radicals and peroxides.
- Cystine: This is a covalently bonded dimer molecule of two cysteine molecules, connected through a disulfide bond. Disulfide bonds between cysteine residues in peptide chains contribute to the 3D structure of proteins. In dietary supplements and food labeling, this compound is often referred to as a conditionally essential amino acid.
- Cysteine-glutathione disulfide (CySSG): This is a mixed disulfide formed by the reaction of glutathione with the oxidized form of cysteine (cystine). It is one of the forms in which cysteine is stored and transported in plasma.
- Glutathione, oxidized (GSSG): This is the oxidized form of glutathione, produced when two glutathione molecules are linked by a disulfide bond. GSSG is reduced back to GSH by the enzyme glutathione reductase, which also requires NADPH as a cofactor.
Overall, these molecules play vital roles in the body’s antioxidant defenses, detoxification of xenobiotics, modulation of redox-controlled signaling pathways, and regulation of cellular proliferation and apoptosis.
These compounds can be retained, separated, and analyzed using a reverse-phase Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended column. The mobile phase for this method consists of water, acetonitrile (MeCN), and Sulfuric acid, which serves as a buffer. This analytical method can be
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cysteine, Cystine, Cysteine-glutathione disulfide, L-Cysteine
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN -10% |
| Buffer | Gradient H2SO4 0.1-0.3%, 10 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
Description
| Class of Compounds | Thiol, Amino acid |
| Analyzing Compounds | Cysteine, Glutathione, reduced, Cystine, Cysteine-glutathione disulfide, Glutathione oxidized |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Cysteine-glutathione disulfide
Cystine
L-Cysteine

HPLC Method For Analysis Of Cysteine and Cystine on Primesep 100 Column
March 10, 2022
Separation type: Liquid Chromatography Mixed-mode
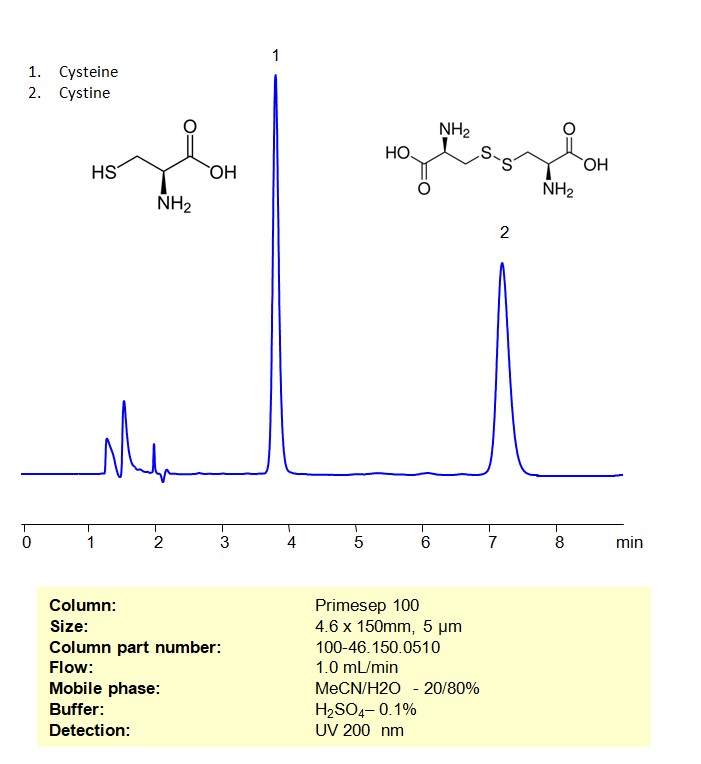
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cysteine and Cystine
Cysteine is an amino acid that is an essential building block of a wide variety of proteins made and used throughout the entire body. Cystine is an oxidized dimer of cysteine that the body uses for redox reactions and as a linkage for proteins to keep their 3D structures. Cysteine and Cystine can be retained and analyzed on a mixed-mode Primesep 100 column with a mobile phase consisting of water, Acetonitrile (MeCN), and Sulfuric acid (H2SO4). This analytical method can be UV detected at 200 nm with high resolution and peak symmetry.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Amino Acid |
| Analyzing Compounds | Cysteine, Cystine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Cystine
L-Cysteine

HPLC Application for Simultaneous Separation of Amino Acids, Hydrophilic Acidic and Hydrophobic Neutral Compounds
December 6, 2007
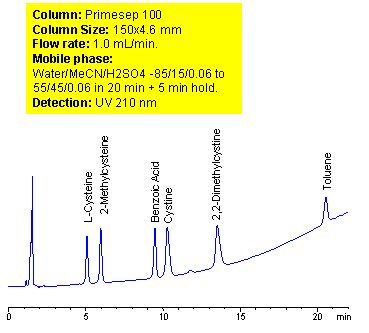
Mixed-mode chromatography allows separating, in single run, compounds with vastly different properties. A method for separation of amino acids (cysteine, methylcysteine, cystine and dimethylcysteine) in the presence of carboxylic acid (benzoic) and hydrophobic neutral compounds was developed on Primesep 100 mixed-mode column. At lower pH ionization of carboxylic acids is suppressed. Amino acids are retained as basic compound based on reverse phase and cation exchange mechanisms. Carboxylic acids are retained on this column based on weak reverse phase mechanisms. Neutral compounds are retained by reverse phase mechanism as on any other column. Retention time of basic, zwitter-ionic and hydrophobic compound can be adjusted by manipulation of mobile phase composition. ELSD, UV or LC/MS detection can be used based on the properties of analytes and mobile phase selection.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Cysteine, Methylcysteine, Cystine, Dimethylcysteine, Benzoic acid, Toluene, |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Methylcysteine
Benzoic Acid
Cystine
L-Cysteine
Toluene