| CAS Number | 33099-14-8 |
|---|---|
| Molecular Formula | C5H11NO2S |
| Molecular Weight | 149.210 |
| InChI Key | LWKUSWORIVQIAU-YFKPBYRVSA-N |
| LogP | -0.404 |
| Synonyms |
|
Applications:
HPLC Application for Simultaneous Separation of Amino Acids, Hydrophilic Acidic and Hydrophobic Neutral Compounds
December 6, 2007
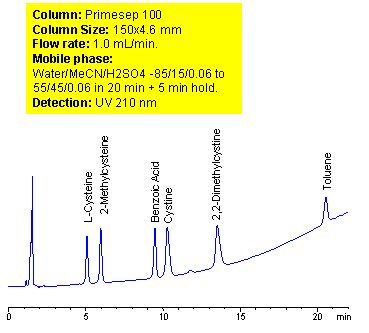
Mixed-mode chromatography allows separating, in single run, compounds with vastly different properties. A method for separation of amino acids (cysteine, methylcysteine, cystine and dimethylcysteine) in the presence of carboxylic acid (benzoic) and hydrophobic neutral compounds was developed on Primesep 100 mixed-mode column. At lower pH ionization of carboxylic acids is suppressed. Amino acids are retained as basic compound based on reverse phase and cation exchange mechanisms. Carboxylic acids are retained on this column based on weak reverse phase mechanisms. Neutral compounds are retained by reverse phase mechanism as on any other column. Retention time of basic, zwitter-ionic and hydrophobic compound can be adjusted by manipulation of mobile phase composition. ELSD, UV or LC/MS detection can be used based on the properties of analytes and mobile phase selection.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Cysteine, Methylcysteine, Cystine, Dimethylcysteine, Benzoic acid, Toluene, |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Methylcysteine
Benzoic Acid
Cystine
L-Cysteine
Toluene

Hydrophobic and Hydrophilic Compound Separation
September 25, 2003
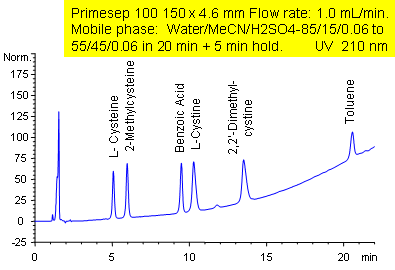
Primesep 100 separates a mixture of polar and nonpolar compounds in one analytical run. The amino acid cysteine; amino acid derivatives L-cystine, 2,2-dimethylcystine, and 2-methylcysteine; the polar acid benzoic acid; and the nonpolar neutral toluene are separated by a gradient using a combination of polar and hydrophobic interactions. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and sulfuric acid (H2SO4) with UV detection at 210 nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Nonpolar, Polar, Supplements |
| Analyzing Compounds | Cysteine, L-cystine, 2,2-dimethylcystine, 2-methylcysteine, Benzoic Acid, Toluene |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Methylcysteine
Amino Acids
Benzoic Acid
Cysteine
L-Cystine
Toluene