| CAS Number | 67-20-9 |
|---|---|
| Molecular Formula | C8H6N4O5 |
| Molecular Weight | 238.159 |
| InChI Key | NXFQHRVNIOXGAQ-UHFFFAOYSA-N |
| LogP | -0.470 |
| Synonyms |
|
Applications:
Application USP Methods for Nitrofurantoin for the Legacy L1
February 20, 2019
HPLC Method for Nitrofurantoin on Legacy L1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Nitrofurantoin.
Nitrofurantoin is an oral antibiotic with the chemical formula C8H6N4O5. It is used to treat urinary tract infections.
Nitrofurantoin can be retained and analyzed using the Legacy L1 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Legacy L1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 43/55% |
| Buffer | NaHPO4 pH3.5 – 2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 233 nm |
| Class of Compounds |
Drug, Acid, Ionizable, Hydrophobic |
| Analyzing Compounds | Nitrofurantoin |
Application Column
Legacy L1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Separation of Antibiotics in Fish Production
August 22, 2008
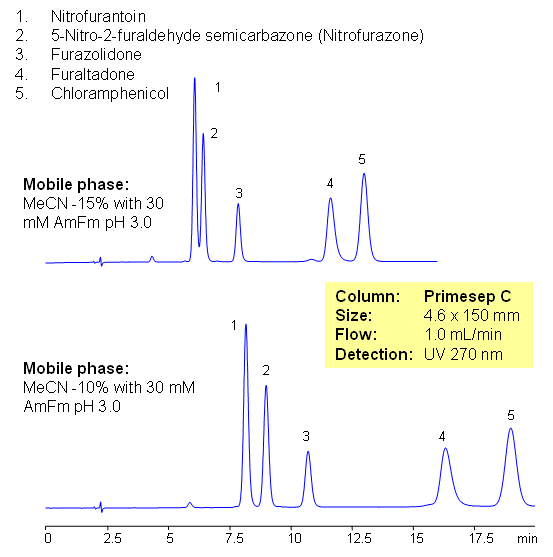
Five antibiotics are separated on Primesep C mixed-mode cation-exchange column using UV, ESLD and LC/MS compatible conditions. Similar compounds can be separated based on reverse phase and cation-exchange mechanisms. Current method can be used in quantitative determination of antibiotics in various food products.
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsChloramphenicol
Furaltadone
Furazolidone
Nitrofurantoin

HPLC Separation of Common Antibiotics in Fish Farming
December 6, 2007
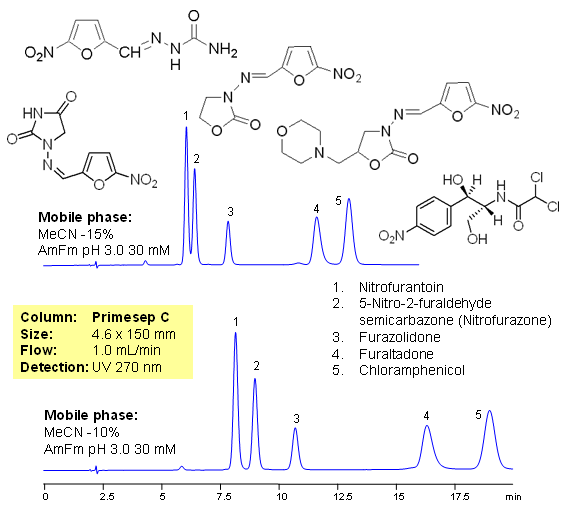
Fish antibiotics are used in fish farming to treat bacterial diseases of fish. It is common practice in the fish industry, particularly in developing countries, to use large amounts of antibiotics to prevent infection. The antibiotics used are often non-biodegradable and remain in the environment for long periods of time, contaminating soil and ground waters. In farming antibiotics are mixed with food and residual amount of drugs ends up in fish products and poultry, this lead to consumption of antibiotics and metabolites by humans. Five common antibiotics are separated on Primesep C column using LC/MS compatible conditions. Method can be used for quantitative determination of nitrofurantoin, nitrofurazone, furazolidone, furaltadone, chloramphenicol and nitrofurazone in fish products and environment.
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsChloramphenicol
Furaltadone
Furazolidone
Nitrofurantoin
Nitrofurazone