| CAS Number | 65277-42-1 |
|---|---|
| Molecular Formula | C26H28Cl2N4O4 |
| Molecular Weight | 531.430 |
| InChI Key | XMAYWYJOQHXEEK-OZXSUGGESA-N |
| LogP | 4.35 |
| Synonyms |
|
Applications:
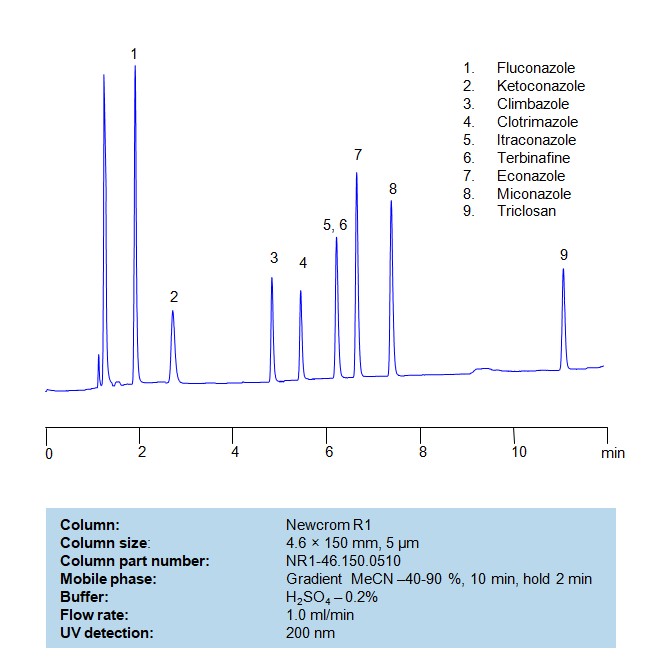
HPLC Method for Separation of a Mixture of Antifungal Agents on Primesep B Column
September 20, 2023
HPLC Method for Analysis of Antifungal Agents on Primesep B by SIELC Technologies
Separation and Analysis of Antifungal Agents on a Primesep B Column Using Gradient HPLC Method
Antifungal agents are drugs used to treat fungal infections. Depending on their mechanism of action and chemical structure, antifungal agents can be categorized into several classes. Here are some of the main classes and examples of antifungal agents:
- Fluconazole: A triazole antifungal mainly used for the treatment and prevention of superficial and systemic fungal infections.
- Ketoconazole: An imidazole antifungal used to treat a wide variety of fungal infections, though its oral use has become less common due to potential side effects. It’s still frequently used topically.
- Climbazole: An imidazole antifungal primarily used in hair care products to treat dandruff.
- Clotrimazole: An imidazole antifungal used to treat various fungal infections including vaginal yeast infections, oral thrush, and ringworm.
- Itraconazole: A triazole antifungal used primarily to treat a variety of systemic fungal infections.
- Terbinafine: This compound belongs to the allylamine class. It’s mainly used to treat fungal infections of the nails and skin, like athlete’s foot and ringworm.
- Econazole: An imidazole antifungal used mainly for skin infections such as athlete’s foot and ringworm.
- Miconazole: An imidazole antifungal with a broad spectrum of activity. It’s used for a variety of skin infections and also as a vaginal cream for yeast infections.
- Triclosan: This is a broad-spectrum antimicrobial agent. While it has some antifungal activity, it’s more commonly known for its antibacterial properties. Due to concerns regarding its safety and potential contribution to antibiotic resistance, its use in hand soaps and some other personal care products has been phased out in several regions.
Of these, fluconazole, itraconazole, ketoconazole, climbazole, clotrimazole, econazole, and miconazole belong to the azole class, which primarily acts by inhibiting the fungal enzyme lanosterol 14α-demethylase. This enzyme is crucial for ergosterol synthesis, a vital component of fungal cell membranes. Terbinafine, on the other hand, inhibits squalene epoxidase, another enzyme important in ergosterol synthesis. Triclosan works through a different mechanism, targeting bacterial and fungal fatty acid synthesis.
Antifungal Agents can be separated, retained, and analyzed on a Primesep B mix mode phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and sulfuric acid as a buffer. This analysis method can be detected in the UV 200 nm.
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-60%, 20 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Antifungal Agents |
| Analyzing Compounds | Fluconazole, Ketoconazole, Climbazole, Clotrimazole, Itraconazole, Terbinafine, Econazole, Miconazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Clotrimazole
Econazole
Fluconazole
Itraconazole
Ketoconazole
Miconazole
Terbinafine

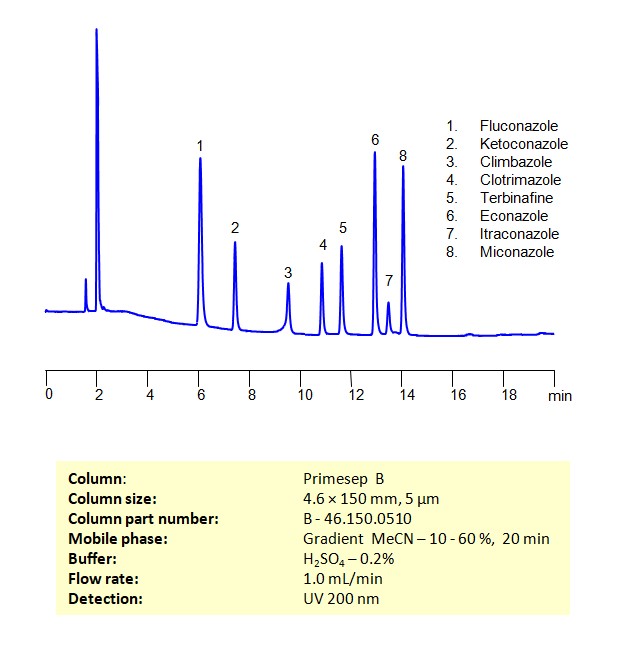
HPLC Method for Separation of a Mixture of Antifungal Agents on Newcrom R1 Column
September 20, 2023
HPLC Method for Analysis of Antifungal Agents on Newcrom R1 by SIELC Technologies
Separation and Analysis of Antifungal Agents on a Newcrom R1 Reverse Phase Column Using Gradient HPLC Method
Antifungal agents are drugs used to treat fungal infections. Depending on their mechanism of action and chemical structure, antifungal agents can be categorized into several classes. Here are some of the main classes and examples of antifungal agents:
- Fluconazole: A triazole antifungal mainly used for the treatment and prevention of superficial and systemic fungal infections.
- Ketoconazole: An imidazole antifungal used to treat a wide variety of fungal infections, though its oral use has become less common due to potential side effects. It’s still frequently used topically.
- Climbazole: An imidazole antifungal primarily used in hair care products to treat dandruff.
- Clotrimazole: An imidazole antifungal used to treat various fungal infections including vaginal yeast infections, oral thrush, and ringworm.
- Itraconazole: A triazole antifungal used primarily to treat a variety of systemic fungal infections.
- Terbinafine: This compound belongs to the allylamine class. It’s mainly used to treat fungal infections of the nails and skin, like athlete’s foot and ringworm.
- Econazole: An imidazole antifungal used mainly for skin infections such as athlete’s foot and ringworm.
- Miconazole: An imidazole antifungal with a broad spectrum of activity. It’s used for a variety of skin infections and also as a vaginal cream for yeast infections.
- Triclosan: This is a broad-spectrum antimicrobial agent. While it has some antifungal activity, it’s more commonly known for its antibacterial properties. Due to concerns regarding its safety and potential contribution to antibiotic resistance, its use in hand soaps and some other personal care products has been phased out in several regions.
Of these, fluconazole, itraconazole, ketoconazole, climbazole, clotrimazole, econazole, and miconazole belong to the azole class, which primarily acts by inhibiting the fungal enzyme lanosterol 14α-demethylase. This enzyme is crucial for ergosterol synthesis, a vital component of fungal cell membranes. Terbinafine, on the other hand, inhibits squalene epoxidase, another enzyme important in ergosterol synthesis. Triclosan works through a different mechanism, targeting bacterial and fungal fatty acid synthesis.
Antifungal agents can be separated, retained, and analyzed on a Newcrom R1 reverse phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and sulfuric acid as a buffer. This analysis method can be detected in the UV 200 nm.
| Column | Newcrom R1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 40-90%, 10 min, hold 2 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Antifungal Agents |
| Analyzing Compounds | Fluconazole, Ketoconazole, Climbazole, Clotrimazole, Itraconazole, Terbinafine, Econazole, Miconazole, Triclosan |
Application Column
Newcrom R1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Clotrimazole
Econazole
Fluconazole
Itraconazole
Ketoconazole
Miconazole
Terbinafine
Triclosan

HPLC Method for Separation of Ketoconazole and Potassium Sorbate on Primesep B Column
February 7, 2023
HPLC Method for Separation of Ketoconazole and Potassium Sorbate on Primesep B by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
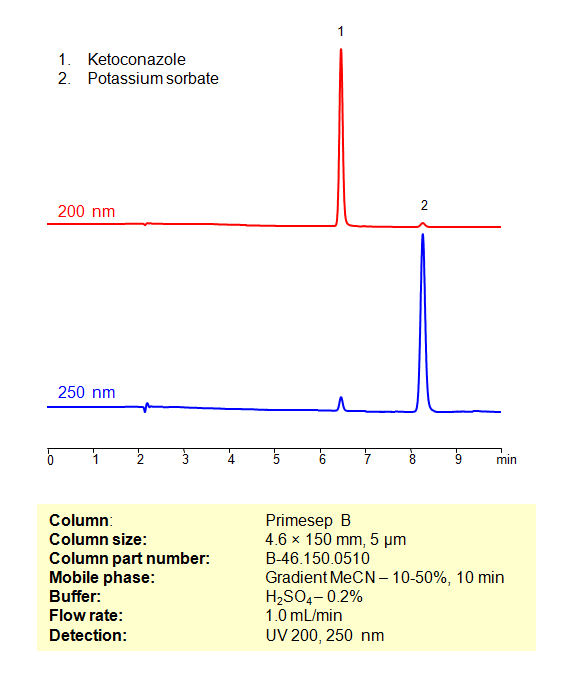
Ketoconazole, also known as Nizoral, is an antifungal compound used to treat dandruff and is commonly available as a shampoo. Ketoconazole can be retained, analyzed, and separated from Potassium sorbate on a Primesep B reverse-phase column using a gradient analytical method with a mobile phase consisting of Acetonitrile (MeCN), water, and Sulfuric acid as the ionic modifier. This analysis method can be UV detected at 200 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ketoconazole and Potassium Sorbate
Condition
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-50%, 10 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200, 250 nm |
| Peak Retention Time | 6.31, 8.12 min |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Potassium sorbate, Sodium sorbate, Ketoconazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Potassium sorbate
Sodium sorbate

HPLC Method for Analysis of Ketoconazole on Primesep B Column
February 7, 2023
HPLC Method for Analysis of Ketoconazole on Primesep B by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
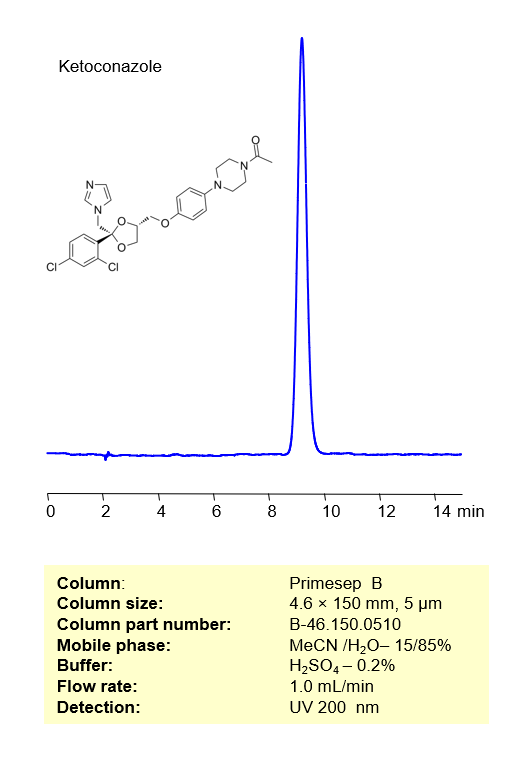
Ketoconazole, also known as Nizoral, is an antifungal compound used to treat dandruff and is commonly available as a shampoo. Ketoconazole can be retained and analyzed on a Primesep B reverse-phase column using a gradient analytical method with a mobile phase consisting of Acetonitrile (MeCN), water, and Sulfuric acid as the ionic modifier. This analysis method can be UV detected at 200 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ketoconazole
Condition
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 15% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Peak Retention Time | 9.32 |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Ketoconazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method for Determination of Ketoconazole in Nizoral Shampoo on Primesep B Column
February 6, 2023
HPLC Method for Determination of Ketoconazole in Nizoral Shampoo on Primesep B by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
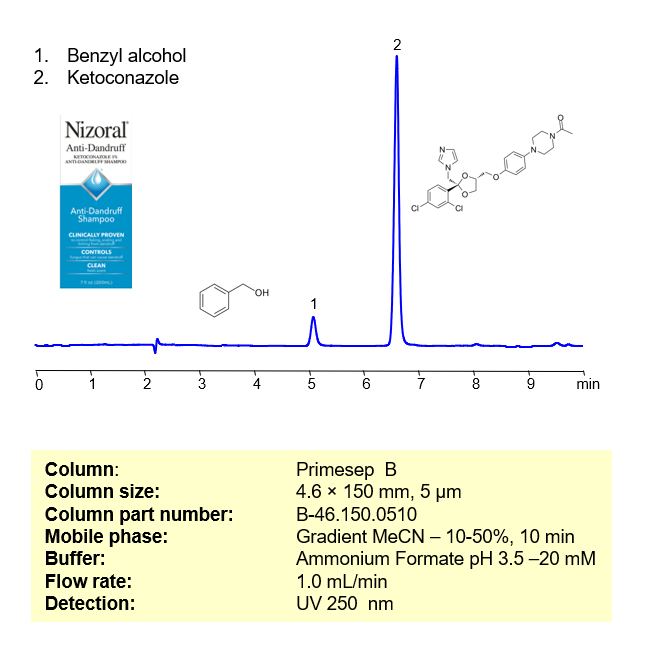
Ketoconazole, also known as Nizoral, is an antifungal compound used to treat dandruff and is commonly available as a shampoo. Ketoconazole can be retained, analyzed, and separated from Benzyl alcohol on a Primesep B reverse-phase column using a gradient analytical method with a mobile phase consisting of Acetonitrile (MeCN), water, and Ammonium formate (AmFm) as the ionic modifier. This analysis method can be UV detected at 250 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ketoconazole
Condition
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-50% |
| Buffer | Ammonium Formate pH 3.5-20 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Peak Retention Time | 6.72 |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Ketoconazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

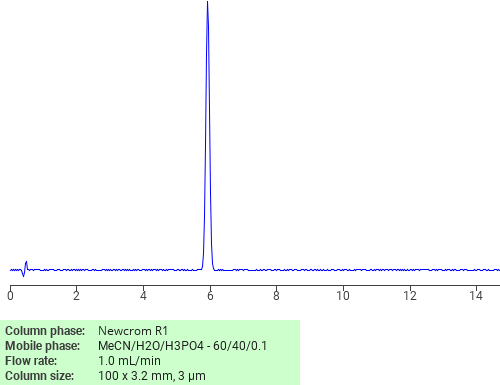
Separation of Ketoconazole on Newcrom R1 HPLC column
February 19, 2018
Ketoconazole can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options