| CAS Number | 50-04-4 |
|---|---|
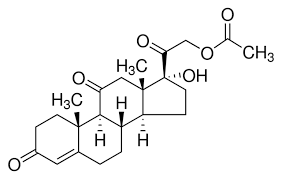
| Molecular Formula | C23H30O6 |
| Molecular Weight | 402.5 |
| InChI Key | ITRJWOMZKQRYTA-RFZYENFJSA-N |
| LogP | 2.1 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Cortisone 21-Acetate on Primesep 100 Column
August 2, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cortisone 21-Acetate on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Cortisone 21-Acetate
Cortisone 21-acetate is a synthetic corticosteroid used in the treatment of various inflammatory and autoimmune conditions. It is the acetate ester of cortisone, which increases its stability and bioavailability when administered.
Mechanism of Action
Cortisone 21-acetate is a prodrug of cortisone, which is converted to its active form, cortisol, in the liver. Cortisol exerts its effects by binding to glucocorticoid receptors, which modulate the expression of anti-inflammatory proteins and inhibit the expression of pro-inflammatory proteins.
Uses
- Anti-inflammatory: Used to treat conditions such as arthritis, lupus, psoriasis, ulcerative colitis, and allergic reactions.
- Immunosuppressive: Employed in the management of autoimmune diseases to suppress the immune response.
- Hormone Replacement Therapy: Used in the treatment of adrenal insufficiency and Addison’s disease.
Cortisone 21-Acetate can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and phosphoric acid as a buffer. This method allows for detection using UV 200 nm.
You can find detailed UV spectra of Cortisone 21-Acetate and information about its various lambda maxima by visiting the following link.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 50% |
| Buffer | H3PO4 -0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Samples | 1.0 mg/ml in MeCN/H2O – 50/50% |
| Injection volume | 1 µl |
| LOD* | 10 ppb (250 nm) |
| Class of Compounds | Synthetic corticosteroid |
| Analyzing Compounds | Cortisone 21-Acetate |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

UV-Vis Spectrum of Cortisone 21-Acetate
July 19, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Cortisone 21-Acetate check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

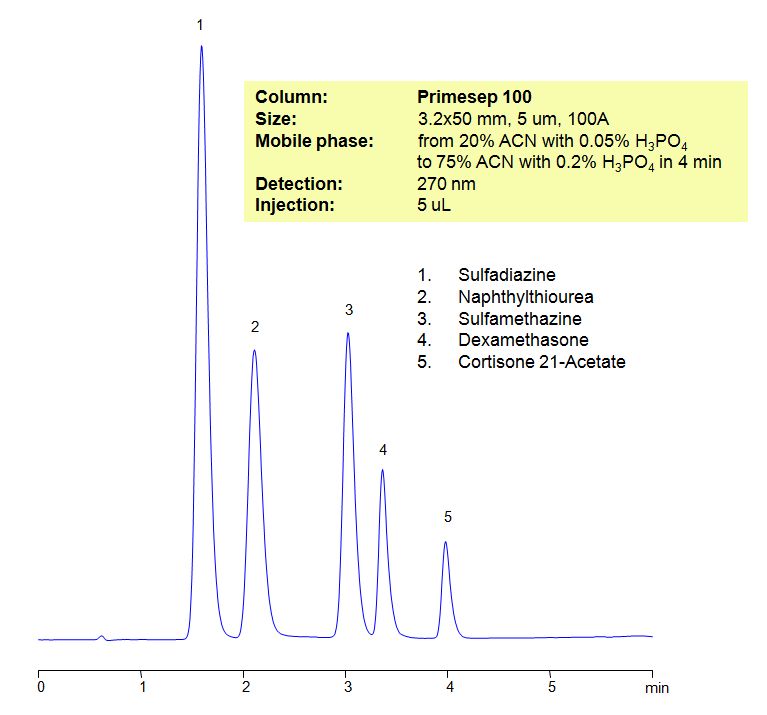
HPLC Method for Analysis of Sulfadiazine, Naphthylthiourea, Sulfamethazine, Dexamethasone, Cortisone 21-Acetate
July 11, 2017
Sulfadiazine is an antibiotic used to prevent rheumatic fever, chancroid, chlamydia and infections by Haemophilus influenzae. There are side effects to the use of the drug which include, but not limited to: nausea, headache, rash, depression.
| Column | Primesep 100, 3.2×50 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 20-75%, 4 min |
| Buffer | Gradient H3PO4 – 0.05-0.2%, 4 min |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Sulfadiazine, Naphthylthiourea, Sulfamethazine, Dexamethasone, Cortisone 21-Acetate |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDexamethasone
Naphthylthiourea
Sulfadiazine
Sulfamethazine