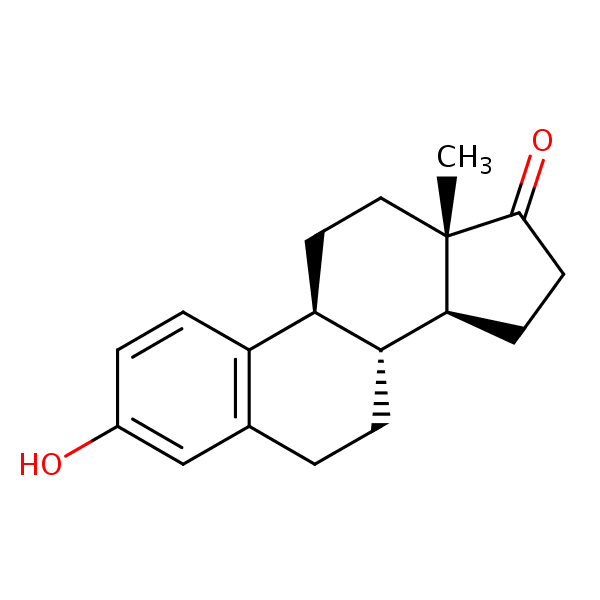
| CAS Number | 53-16-7 |
|---|---|
| Molecular Formula | C18H22O2 |
| Molecular Weight | 270.373 |
| InChI Key | DNXHEGUUPJUMQT-CBZIJGRNSA-N |
| LogP | 3.13 |
| Synonyms |
|
Applications:
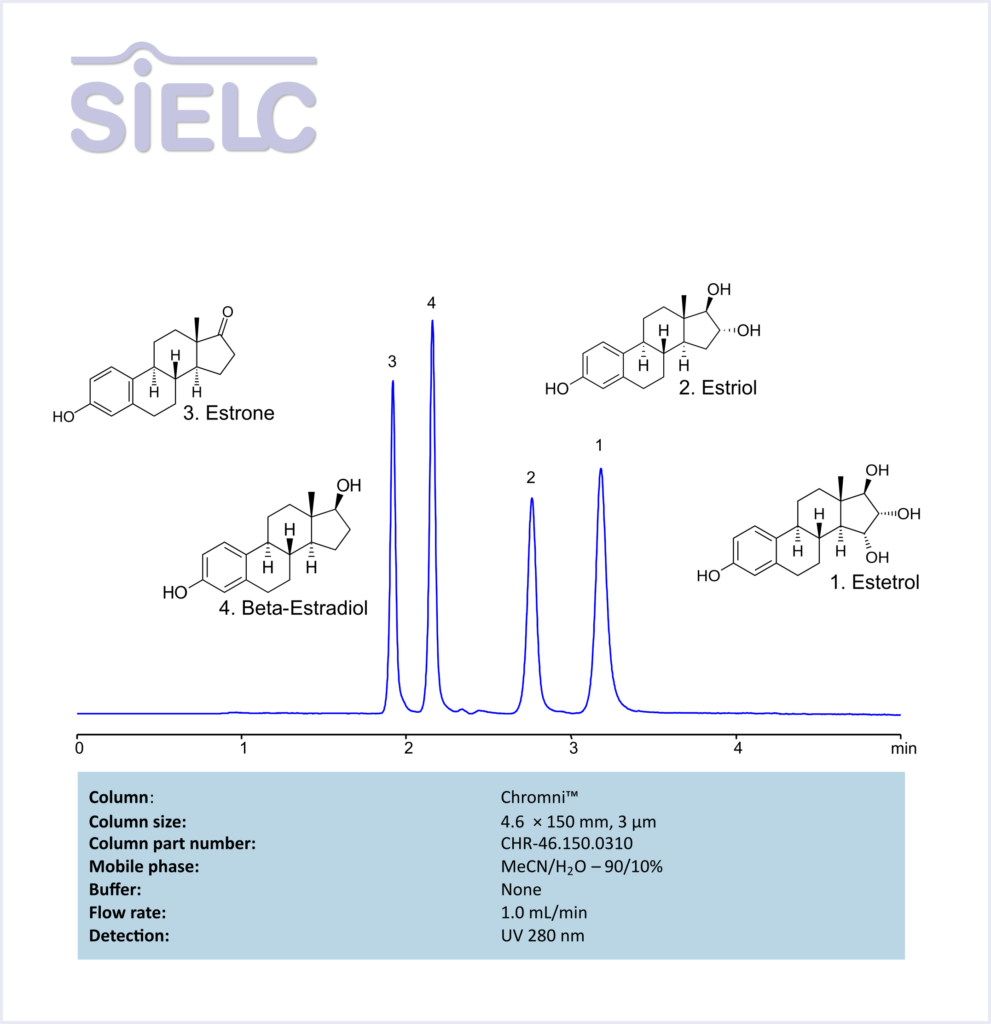
HPLC Method for Analysis of Estetrol, Estiol, Estrone, and Besta-Estradiol Without Buffer on Chromni Column
September 16, 2025
HPLC Method for Estrone, Estradiol, Estriol, Estetrol on Chromni™ by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Estrone, Estradiol, Estriol, Estetrol
Estetrol is an estrogenic steroid hormone compound with the molecular formula C18H24O4.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in water.
Uses: Production of contraceptives.
Estriol is an estrogenic steroid hormone compound with the molecular formula C18H24O3.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~288.4 g/mol
Solubility: Soluble in benzene, water, alcohol, and vegetable oils.
Uses: Hormone Replacement Therapy and similar medication.
Estrone is an synthetic estrogenic steroid hormone with the molecular formula C18H22O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in benzene, water, acetone, and vegetable oils.
Uses: Production of medication
Beta-Estradiol is an estrogenic steroid hormone compound with the molecular formula C18H24O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~272.4 g/mol
Solubility: Soluble in ethanol, water, and vegetable oils.
Uses: Production of medication for treatment of menopausal symptoms
Estrone, Estradiol, Estriol, Estetrol can be retained and analyzed using the Chromni™ stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN). Detection is performed using UV.
| Column | Chromni™, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 90% |
| Buffer | None |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm |
Application Column
Chromni™
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Estradiol
Estriol
Estrone

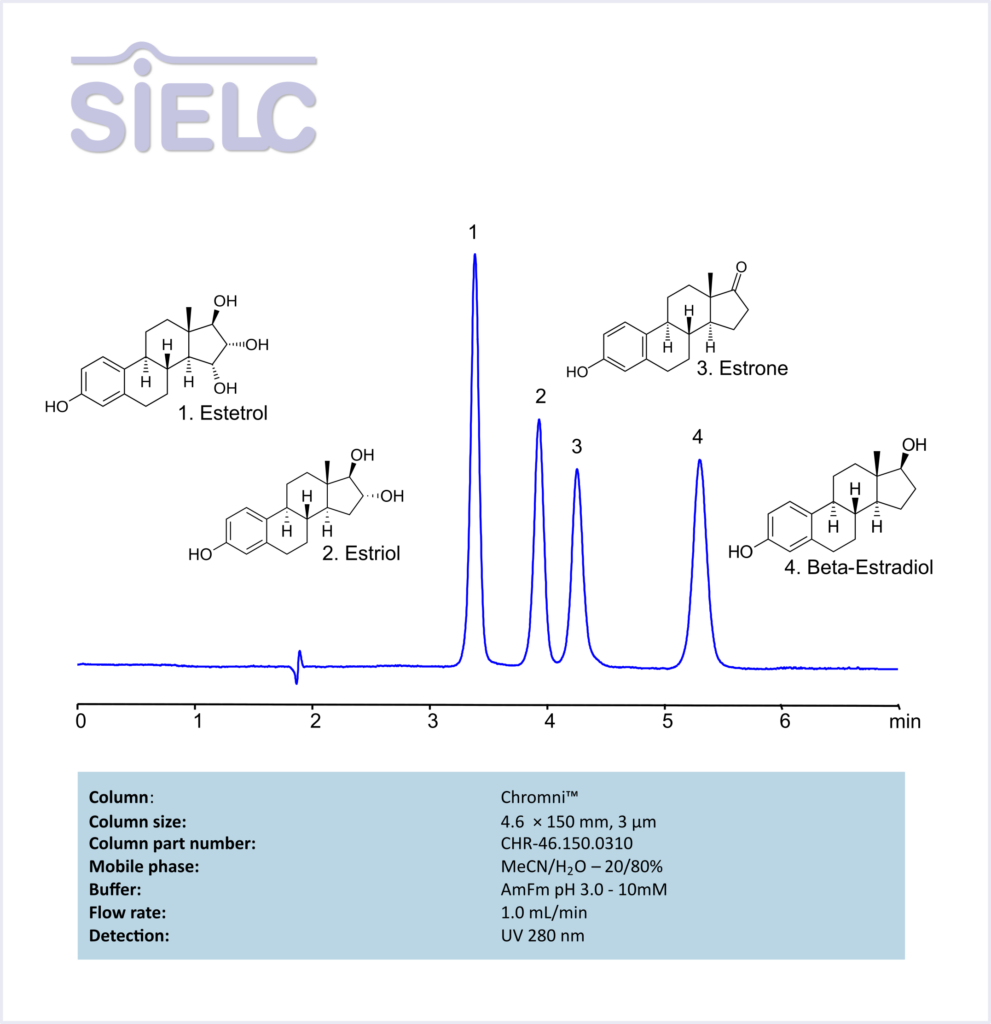
HPLC Method for Analysis of Estetrol, Estiol, Estrone, and Besta-Estradiol on Chromni Column
September 16, 2025
HPLC Method for Estrone, Estradiol, Estetrol, Estriol on Chromni™ by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Estrone, Estradiol, Estetrol, Estriol
Estetrol is an synthetic organochlorine compound with the molecular formula C18H24O4.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in water.
Uses: Production of contraceptives.
Estriol is an synthetic organochlorine compound with the molecular formula C18H24O3.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~288.4 g/mol
Solubility: Soluble in benzene, water, alcohol, and vegetable oils.
Uses: Hormone Replacement Therapy and similar medication.
Estrone is an synthetic organochlorine compound with the molecular formula C18H22O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in benzene, water, acetone, and vegetable oils.
Uses: Production of medication
Beta-Estradiol is an synthetic organochlorine compound with the molecular formula C18H24O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~272.4 g/mol
Solubility: Soluble in ethanol, water, and vegetable oils.
Uses: Production of medication for treatment of menopausal symptoms
Estrone, Estradiol, Estetrol, Estriol can be retained and analyzed using the Chromni™ stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and ammonium formate. Detection is performed using UV.
| Column | Chromni™, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | Ammonium Formate – 10mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm |
Application Column
Chromni™
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Estradiol
Estriol
Estrone

HPLC Separation of Estrone
September 9, 2015
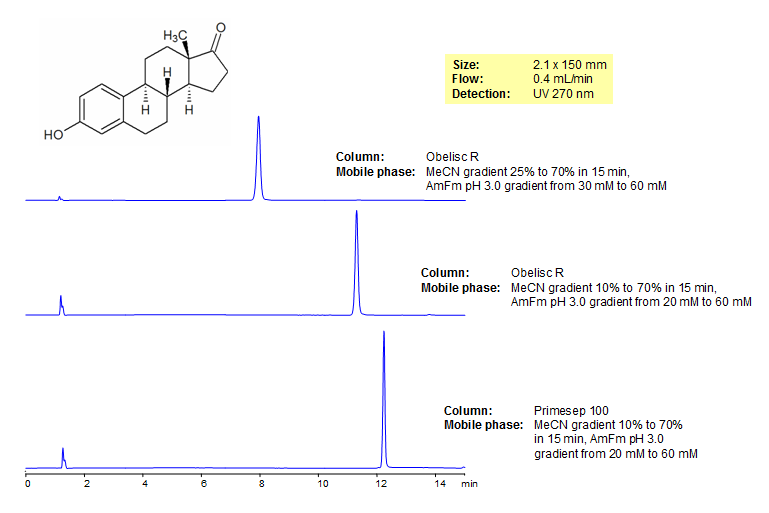
Estrone, also known at oestrone and E1 is a naturally occurring estrogen which is the predominant estrogen in postmenopausal women. It can be found in its long-lived form estrone sulfate. In certain scenarios estrone can be a carcinogen and causes breast pain, nausea, headaches and leg cramps. It was separated on both Obelisc R and Primesep 100 which have unique modes of retention. Method is LC/MS compatible and useful for separating a number of pesticides.
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 25-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 30-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Primesep 100, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Hormone, Hydrophobic, Ionizable |
| Analyzing Compounds | Estrone |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsPrimesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options