| CAS Number | 53-16-7 |
|---|---|
| Molecular Formula | C18H22O2 |
| Molecular Weight | 270.373 |
| InChI Key | DNXHEGUUPJUMQT-CBZIJGRNSA-N |
| LogP | 3.13 |
| Synonyms |
|
Applications:
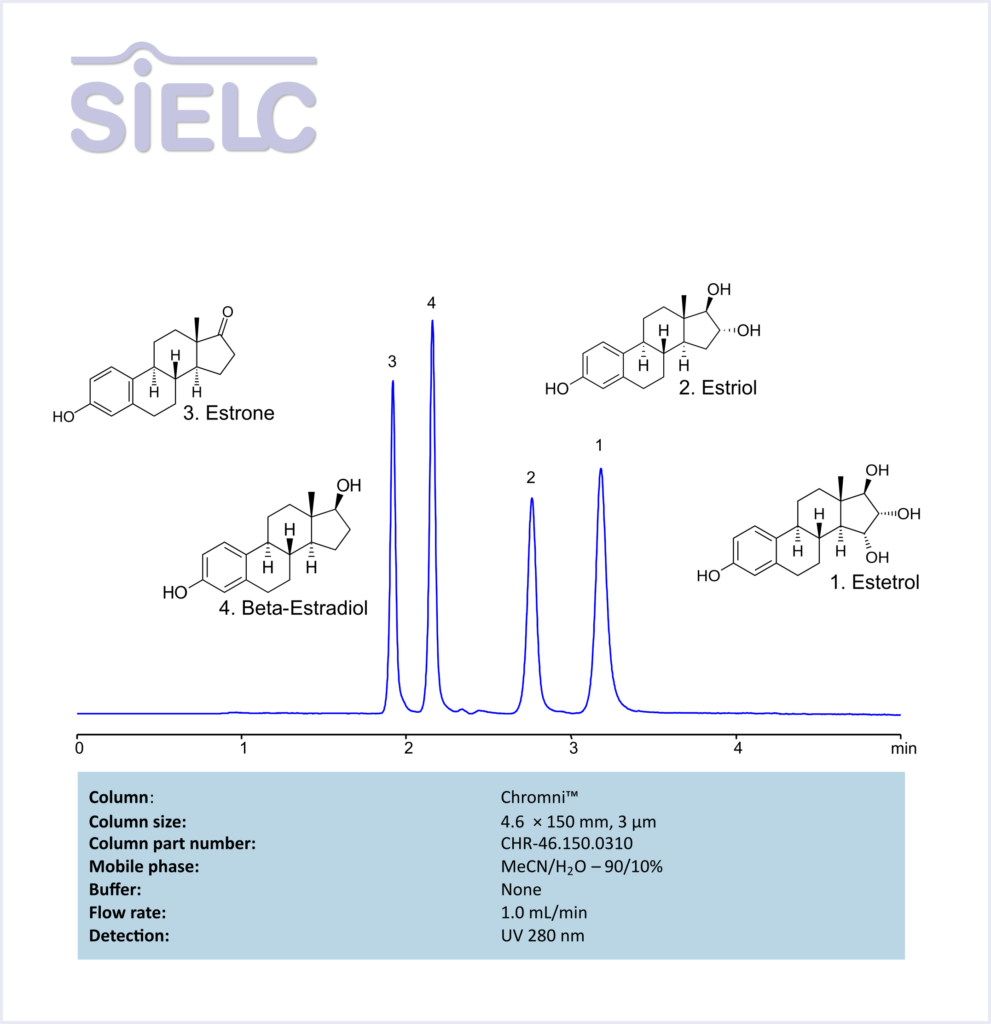
HPLC Method for Analysis of Estetrol, Estiol, Estrone, and Besta-Estradiol Without Buffer on Chromni Column
September 16, 2025
HPLC Method for Estrone, Estradiol, Estriol, Estetrol on Chromni by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Estrone, Estradiol, Estriol, Estetrol
Estetrol is an estrogenic steroid hormone compound with the molecular formula C18H24O4.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in water.
Uses: Production of contraceptives.
Estriol is an estrogenic steroid hormone compound with the molecular formula C18H24O3.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~288.4 g/mol
Solubility: Soluble in benzene, water, alcohol, and vegetable oils.
Uses: Hormone Replacement Therapy and similar medication.
Estrone is an synthetic estrogenic steroid hormone with the molecular formula C18H22O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in benzene, water, acetone, and vegetable oils.
Uses: Production of medication
Beta-Estradiol is an estrogenic steroid hormone compound with the molecular formula C18H24O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~272.4 g/mol
Solubility: Soluble in ethanol, water, and vegetable oils.
Uses: Production of medication for treatment of menopausal symptoms
Estrone, Estradiol, Estriol, Estetrol can be retained and analyzed using the Chromni stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN). Detection is performed using UV.
| Column | Chromni, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 90% |
| Buffer | None |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm |
Application Column
Chromni
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Estradiol
Estriol
Estrone

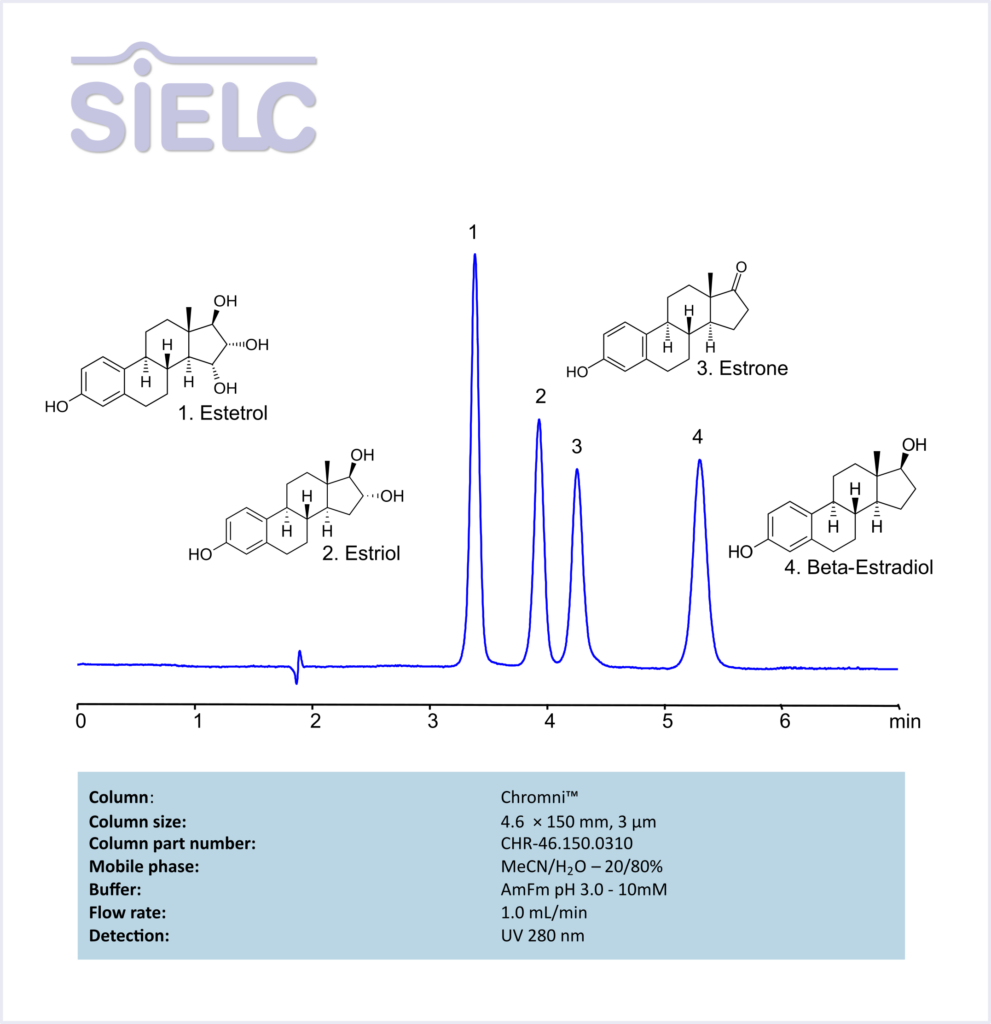
HPLC Method for Analysis of Estetrol, Estiol, Estrone, and Besta-Estradiol on Chromni Column
September 16, 2025
HPLC Method for Estrone, Estradiol, Estetrol, Estriol on Chromni by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Estrone, Estradiol, Estetrol, Estriol
Estetrol is an synthetic organochlorine compound with the molecular formula C18H24O4.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in water.
Uses: Production of contraceptives.
Estriol is an synthetic organochlorine compound with the molecular formula C18H24O3.
Properties:
Appearance: Typically a white, odorless, powder.
Molecular weight: ~288.4 g/mol
Solubility: Soluble in benzene, water, alcohol, and vegetable oils.
Uses: Hormone Replacement Therapy and similar medication.
Estrone is an synthetic organochlorine compound with the molecular formula C18H22O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~270.4 g/mol
Solubility: Soluble in benzene, water, acetone, and vegetable oils.
Uses: Production of medication
Beta-Estradiol is an synthetic organochlorine compound with the molecular formula C18H24O2.
Properties:
Appearance: Typically a white, odorless, crystals.
Molecular weight: ~272.4 g/mol
Solubility: Soluble in ethanol, water, and vegetable oils.
Uses: Production of medication for treatment of menopausal symptoms
Estrone, Estradiol, Estetrol, Estriol can be retained and analyzed using the Chromni stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and ammonium formate. Detection is performed using UV.
| Column | Chromni, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | Ammonium Formate – 10mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm |
Application Column
Chromni
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Estradiol
Estriol
Estrone

HPLC Separation of Estrone
September 9, 2015
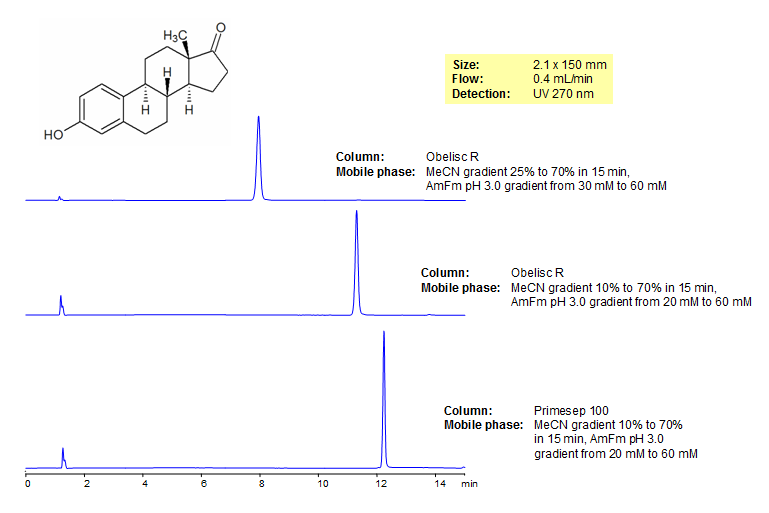
Estrone, also known at oestrone and E1 is a naturally occurring estrogen which is the predominant estrogen in postmenopausal women. It can be found in its long-lived form estrone sulfate. In certain scenarios estrone can be a carcinogen and causes breast pain, nausea, headaches and leg cramps. It was separated on both Obelisc R and Primesep 100 which have unique modes of retention. Method is LC/MS compatible and useful for separating a number of pesticides.
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 25-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 30-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Primesep 100, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM, 15 min |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Hormone, Hydrophobic, Ionizable |
| Analyzing Compounds | Estrone |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsPrimesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options