| CAS Number | 92-67-1 |
|---|---|
| Molecular Formula | C12H11N |
| Molecular Weight | 169.228 |
| InChI Key | DMVOXQPQNTYEKQ-UHFFFAOYSA-N |
| LogP | 2.86 |
| Synonyms |
|
Applications:
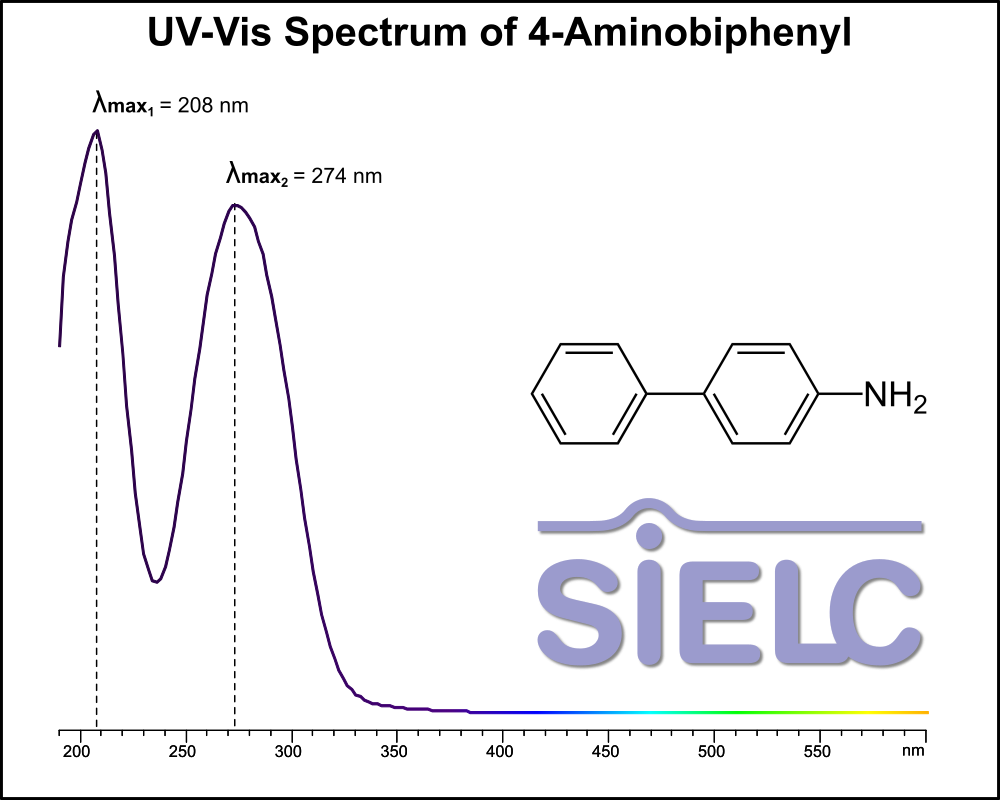
Uv-Vis Spectrum of 4-Aminobiphenyl
February 20, 2026
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze 4-Aminobiphenyl check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

Separation of Toluidine and Aminobiphenyl Isomers
July 6, 2015
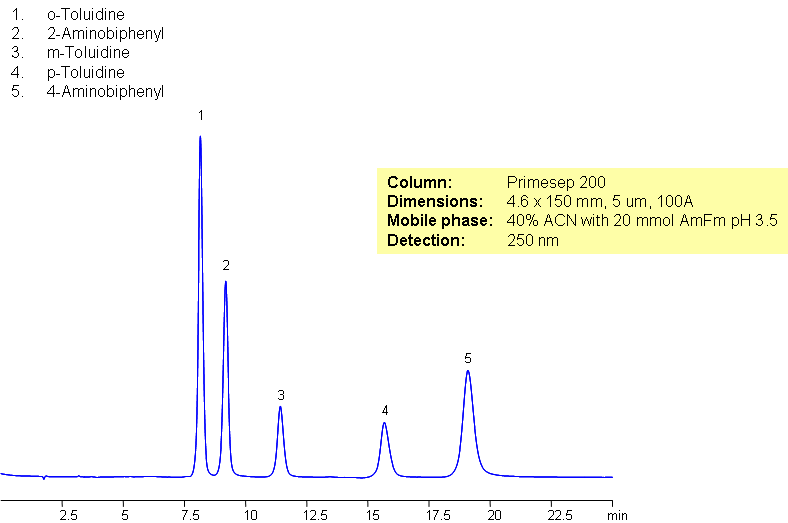 Toluidine isomers and aminobyphenyl isomers are organic compounds each with a substituted amine group. Primesep 200 was used to separate these isomers. Primesep 200 separates isomers by interacting with polar molecules, retaining basic compounds, and additionally by reverse phase mechanism.
Toluidine isomers and aminobyphenyl isomers are organic compounds each with a substituted amine group. Primesep 200 was used to separate these isomers. Primesep 200 separates isomers by interacting with polar molecules, retaining basic compounds, and additionally by reverse phase mechanism.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm Ph 3.5 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Hydrophobic, Ionizable |
| Analyzing Compounds | o-Toluidine, 2-Aminobiphenyl, m-Toluidine, p-Toluidine, 4-Aminobiphenyl |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options4-Aminobiphenyl
m-Toluidine
o-Toluidine
p-Toluidine

HPLC Separation of Aminobiphenyl Isomers in Cation-Exchange Mode
July 14, 2011
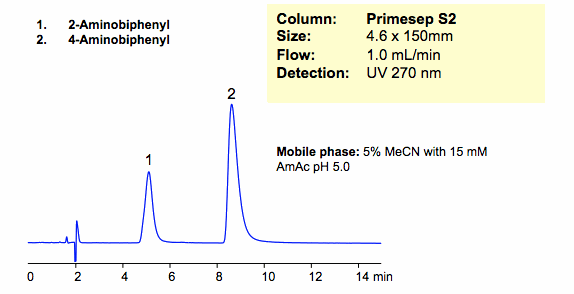
Two isomers of aminobiphenyl were successfully retained and separated in cation-exchange mode on a Primesep S2 column. Primesep S2 column is a HILIC/cation-exchange column that can be operated in HILIC, cation-exchange, and anion-exclusion modes. Method and column are compatible with ELSD/LC/MS and UV and can be used for routine analysis of polar molecules.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | AmAc pH 5.0 15 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Hydrophobic, Ionizable |
| Analyzing Compounds | 2-Aminobiphenyl, 4 -Aminobiphenyl |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options4-Aminobiphenyl

HPLC Separation of Aminobiphenyls
April 10, 2005
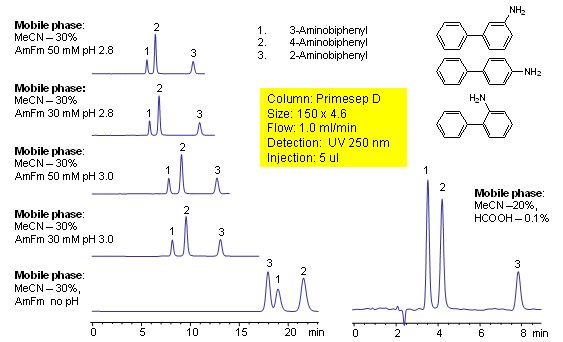
Aminobiphenyls consist of isomers that differ only in the location of a primary amine group on an aromatic ring. 2-, 3-, and 4-aminobiphenyl are separated on a Primesep D with good peak shape and a short retention time by a mixture of reversed-phase and ion-exclusion interactions. Peak order can be reversed for 2- and 3-aminobiphenyls by using ammonium formate buffer without pH adjustment. The HPLC separations use a mobile phase of water, acetonitrile (MeCN, ACN), ammonium formate buffer or acetic acid (HOAc) with UV detection at 250 nm.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | Formic Acid, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Hydrophobic, Ionizable |
| Analyzing Compounds | 2-Aminobiphenyl, 4 -Aminobiphenyl, 3 -Aminobiphenyl |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Aminobiphenyl
4-Aminobiphenyl