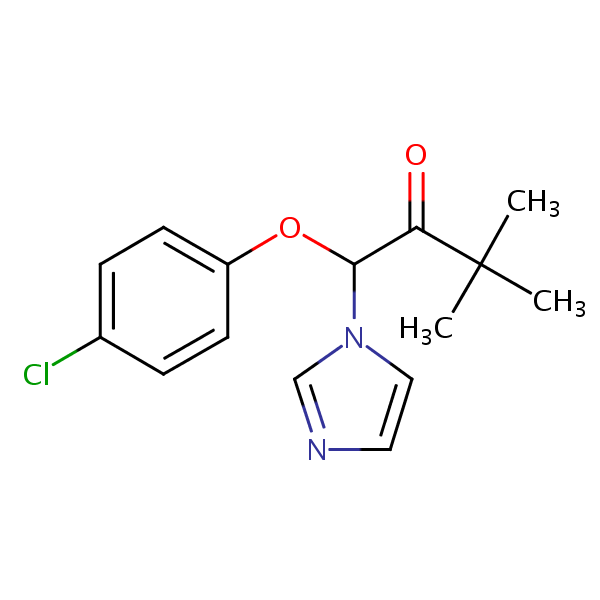
| CAS Number | 38083-17-9 |
|---|---|
| Molecular Formula | C15H17ClN2O2 |
| Molecular Weight | 292.761 |
| InChI Key | OWEGWHBOCFMBLP-UHFFFAOYSA-N |
| LogP | 3.24 |
| Synonyms |
|
Applications:
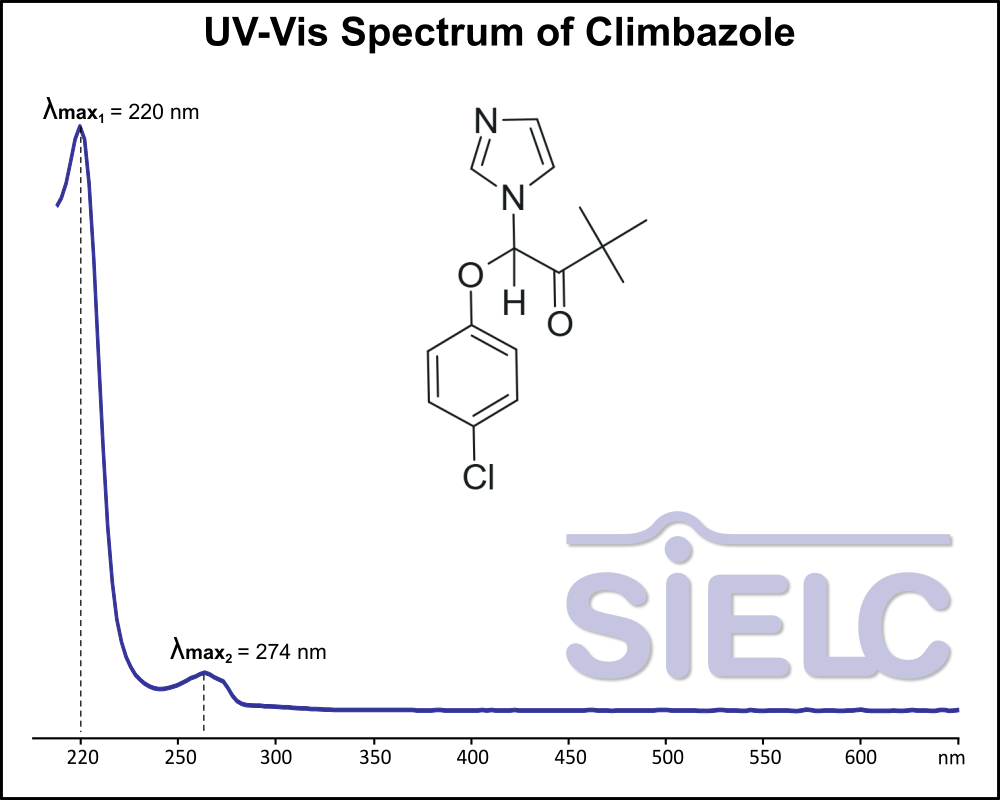
UV-Vis Spectrum of Climbazole
July 31, 2025
If you are looking for optimized HPLC method to analyze Climbazole check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Separation of a Mixture of Antifungal Agents on Primesep B Column
September 20, 2023
HPLC Method for Analysis of Antifungal Agents on Primesep B by SIELC Technologies
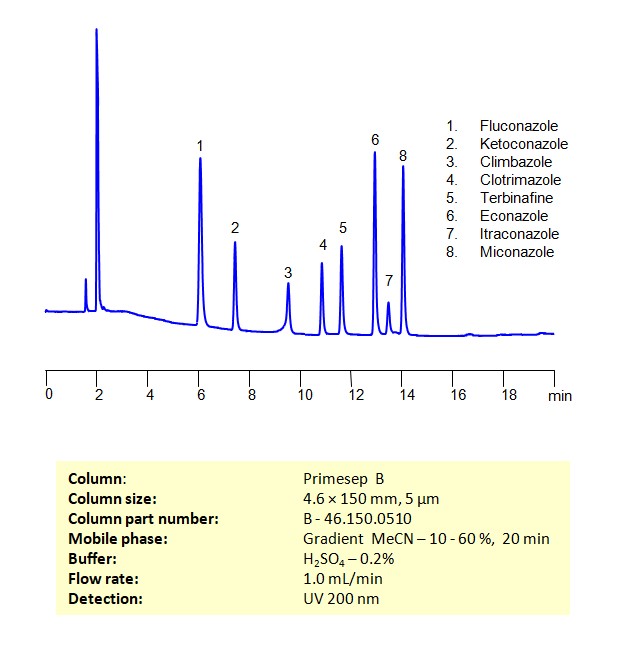
Separation and Analysis of Antifungal Agents on a Primesep B Column Using Gradient HPLC Method
Antifungal agents are drugs used to treat fungal infections. Depending on their mechanism of action and chemical structure, antifungal agents can be categorized into several classes. Here are some of the main classes and examples of antifungal agents:
- Fluconazole: A triazole antifungal mainly used for the treatment and prevention of superficial and systemic fungal infections.
- Ketoconazole: An imidazole antifungal used to treat a wide variety of fungal infections, though its oral use has become less common due to potential side effects. It’s still frequently used topically.
- Climbazole: An imidazole antifungal primarily used in hair care products to treat dandruff.
- Clotrimazole: An imidazole antifungal used to treat various fungal infections including vaginal yeast infections, oral thrush, and ringworm.
- Itraconazole: A triazole antifungal used primarily to treat a variety of systemic fungal infections.
- Terbinafine: This compound belongs to the allylamine class. It’s mainly used to treat fungal infections of the nails and skin, like athlete’s foot and ringworm.
- Econazole: An imidazole antifungal used mainly for skin infections such as athlete’s foot and ringworm.
- Miconazole: An imidazole antifungal with a broad spectrum of activity. It’s used for a variety of skin infections and also as a vaginal cream for yeast infections.
- Triclosan: This is a broad-spectrum antimicrobial agent. While it has some antifungal activity, it’s more commonly known for its antibacterial properties. Due to concerns regarding its safety and potential contribution to antibiotic resistance, its use in hand soaps and some other personal care products has been phased out in several regions.
Of these, fluconazole, itraconazole, ketoconazole, climbazole, clotrimazole, econazole, and miconazole belong to the azole class, which primarily acts by inhibiting the fungal enzyme lanosterol 14α-demethylase. This enzyme is crucial for ergosterol synthesis, a vital component of fungal cell membranes. Terbinafine, on the other hand, inhibits squalene epoxidase, another enzyme important in ergosterol synthesis. Triclosan works through a different mechanism, targeting bacterial and fungal fatty acid synthesis.
Antifungal Agents can be separated, retained, and analyzed on a Primesep B mix mode phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and sulfuric acid as a buffer. This analysis method can be detected in the UV 200 nm.
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-60%, 20 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Antifungal Agents |
| Analyzing Compounds | Fluconazole, Ketoconazole, Climbazole, Clotrimazole, Itraconazole, Terbinafine, Econazole, Miconazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Clotrimazole
Econazole
Fluconazole
Itraconazole
Ketoconazole
Miconazole
Terbinafine

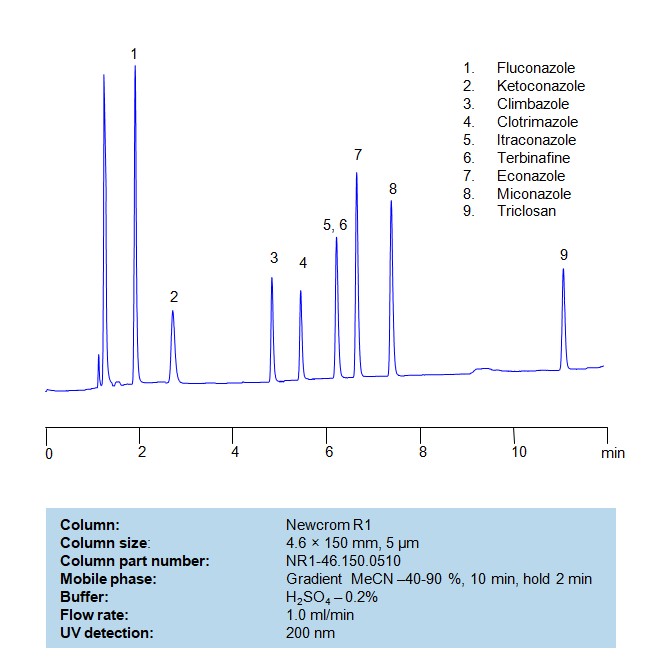
HPLC Method for Separation of a Mixture of Antifungal Agents on Newcrom R1 Column
September 20, 2023
HPLC Method for Analysis of Antifungal Agents on Newcrom R1 by SIELC Technologies
Separation and Analysis of Antifungal Agents on a Newcrom R1 Reverse Phase Column Using Gradient HPLC Method
Antifungal agents are drugs used to treat fungal infections. Depending on their mechanism of action and chemical structure, antifungal agents can be categorized into several classes. Here are some of the main classes and examples of antifungal agents:
- Fluconazole: A triazole antifungal mainly used for the treatment and prevention of superficial and systemic fungal infections.
- Ketoconazole: An imidazole antifungal used to treat a wide variety of fungal infections, though its oral use has become less common due to potential side effects. It’s still frequently used topically.
- Climbazole: An imidazole antifungal primarily used in hair care products to treat dandruff.
- Clotrimazole: An imidazole antifungal used to treat various fungal infections including vaginal yeast infections, oral thrush, and ringworm.
- Itraconazole: A triazole antifungal used primarily to treat a variety of systemic fungal infections.
- Terbinafine: This compound belongs to the allylamine class. It’s mainly used to treat fungal infections of the nails and skin, like athlete’s foot and ringworm.
- Econazole: An imidazole antifungal used mainly for skin infections such as athlete’s foot and ringworm.
- Miconazole: An imidazole antifungal with a broad spectrum of activity. It’s used for a variety of skin infections and also as a vaginal cream for yeast infections.
- Triclosan: This is a broad-spectrum antimicrobial agent. While it has some antifungal activity, it’s more commonly known for its antibacterial properties. Due to concerns regarding its safety and potential contribution to antibiotic resistance, its use in hand soaps and some other personal care products has been phased out in several regions.
Of these, fluconazole, itraconazole, ketoconazole, climbazole, clotrimazole, econazole, and miconazole belong to the azole class, which primarily acts by inhibiting the fungal enzyme lanosterol 14α-demethylase. This enzyme is crucial for ergosterol synthesis, a vital component of fungal cell membranes. Terbinafine, on the other hand, inhibits squalene epoxidase, another enzyme important in ergosterol synthesis. Triclosan works through a different mechanism, targeting bacterial and fungal fatty acid synthesis.
Antifungal agents can be separated, retained, and analyzed on a Newcrom R1 reverse phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and sulfuric acid as a buffer. This analysis method can be detected in the UV 200 nm.
| Column | Newcrom R1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 40-90%, 10 min, hold 2 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Antifungal Agents |
| Analyzing Compounds | Fluconazole, Ketoconazole, Climbazole, Clotrimazole, Itraconazole, Terbinafine, Econazole, Miconazole, Triclosan |
Application Column
Newcrom R1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Clotrimazole
Econazole
Fluconazole
Itraconazole
Ketoconazole
Miconazole
Terbinafine
Triclosan

HPLC Method for Determination of Climbazole in Dandruff Shampoo on Primesep B Column
September 11, 2023
HPLC Method for Analysis of Climbazole on Primesep B by SIELC Technologies
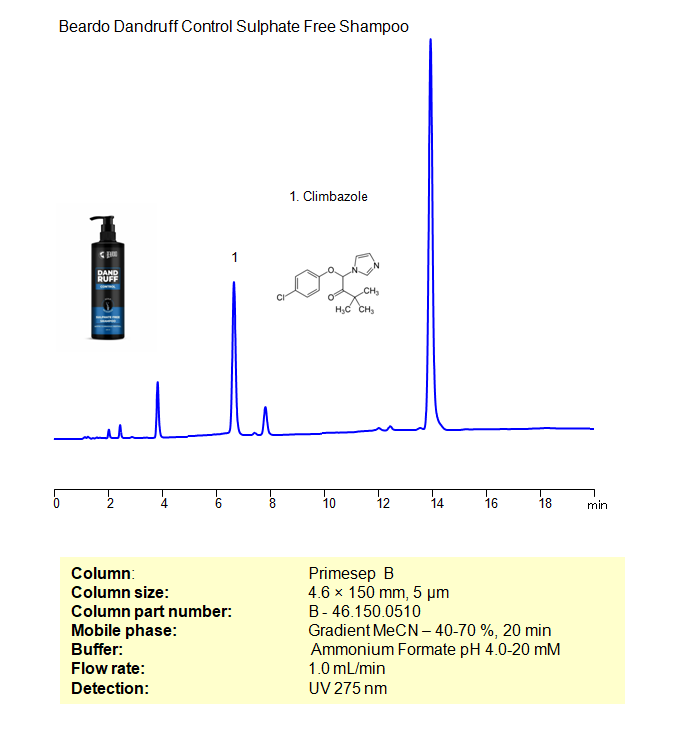
High Performance Liquid Chromatography (HPLC) Method for Analysis of Climbazole
Climbazole is an antifungal agent commonly used in hair care products, primarily in shampoos, to treat dandruff and seborrheic dermatitis. It works by inhibiting the growth of the fungi that contribute to these conditions. Here are some key points about climbazole:
- Mechanism of Action:
- Climbazole is believed to inhibit the growth of fungi by interfering with ergosterol biosynthesis, which is a crucial component of fungal cell membranes. Without ergosterol, the fungal cell membrane becomes compromised, leading to the death of the fungus.
- Applications:
- Hair Care: The primary use of climbazole is in anti-dandruff shampoos and hair lotions. Dandruff and seborrheic dermatitis can be, in part, attributed to the overgrowth of the yeast Malassezia on the scalp. Climbazole helps control the proliferation of this yeast, thereby alleviating the symptoms associated with its overgrowth.
- Skincare: Occasionally, climbazole can also be found in skincare products designed for fungal-related skin conditions.
Climbazole can be retained, and analyzed on a Primesep B mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a ammonium format a buffer. This analysis method can be detected in the UV 270 nm.
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 40-70%, 20 min |
| Buffer | Ammonium Formate pH 4.0-20 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds | Allylamines |
| Analyzing Compounds | Climbazole |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended