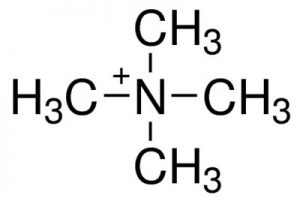
| CAS Number | 51-92-3 |
|---|---|
| Molecular Formula | C4H12N |
| Molecular Weight | 74.146 |
| InChI Key | QEMXHQIAXOOASZ-UHFFFAOYSA-N |
| LogP | -2.41 |
| Synonyms |
|
Applications:
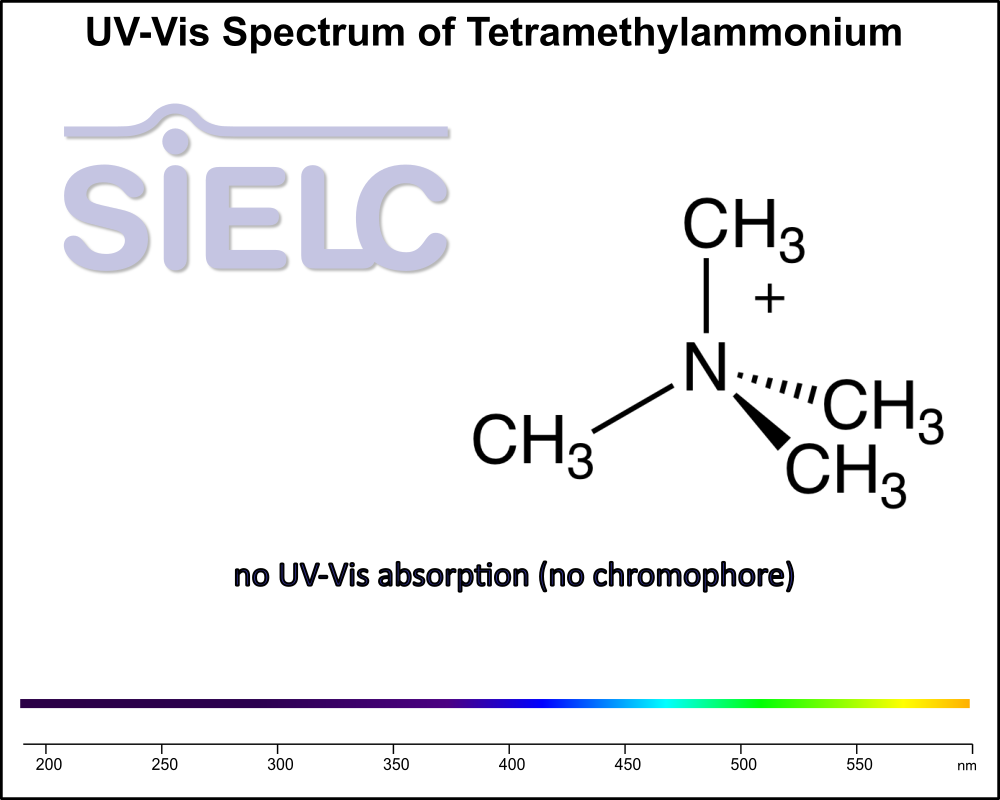
Uv-Vis Spectrum of Tetramethylammonium
February 12, 2026
If you are looking for optimized HPLC method to analyze Tetramethylammonium check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

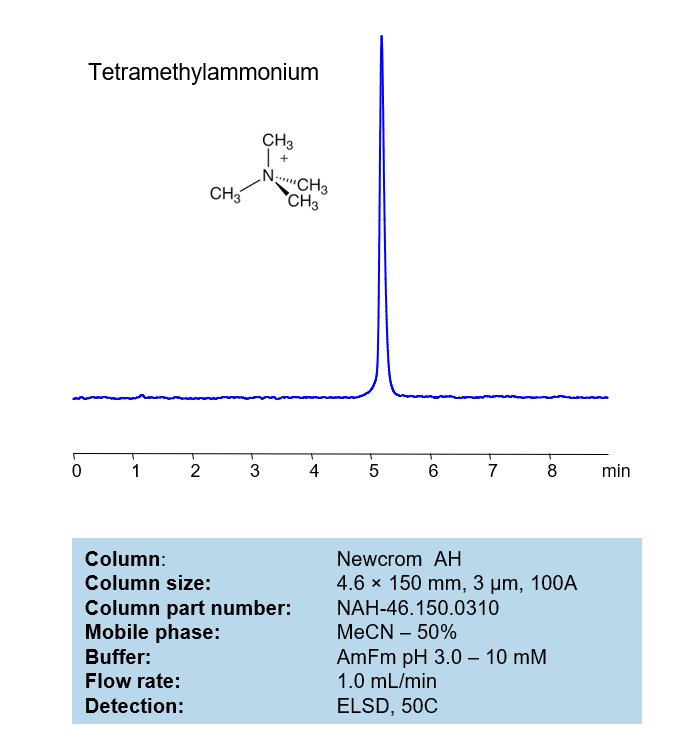
HPLC Method for Analysis of Tetramethylammonium on Newcrom AH Column
July 11, 2023
HPLC Method for Analysis of Tetramethylammonium on (Column not found) by SIELC Technologies
Tetramethylammonium is a quaternary ammonium cation with the formula (CH3)4N+. This compound is one of the simplest quaternary ammonium ions, and is notable for its prevalence in both science and industry.
The central nitrogen atom in tetramethylammonium has a positive charge due to the four alkyl (methyl, CH3) groups attached to it. This makes it a cation, meaning it carries a positive charge.
This compound is often used in its chloride salt form (tetramethylammonium chloride), which is a white crystalline powder that is highly soluble in water and used in various chemical applications, such as phase transfer catalysts, surfactants, and reagents in organic synthesis.
Tetramethylammonium ions can also serve as counter ions or blocking ions in certain biological and chemical studies. For example, they are sometimes used to block potassium channels in studies of cellular physiology.
The tetramethylammonium can be retained, analyzed, on a Newcrom AH column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and an Ammonium formate (AmFm) ionic modifier. This analysis method can be detected with an Evaporative Light Scattering Detector (ELSD) or any other evaporative detection method (CAD, ESI-MS).
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tetramethylammonium
Condition
| Column | (Column variation not found) |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | AmFm pH 3.0- 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, the nebulizer and evaporator temperatures 50 °C, with a gas flow rate of 1.6 Standard Liters per Minute (SLM) (MS- compatible mobile phase) |
| Peak Retention Time | 5.12 min |
| Sample concentration | 1 mg/ml |
| Injection volume | 1 µl |
| LOD | 320 ppb |
Description
| Class of Compounds | Hydrophilic, Metal, Ion, Quaternary ammonium salt |
| Analyzing Compounds | Tetramethylammonium |

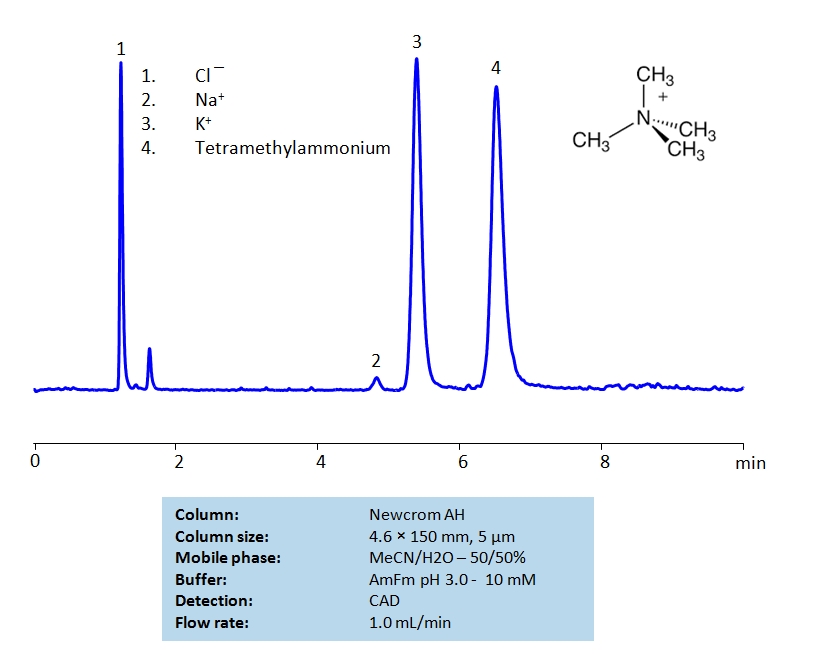
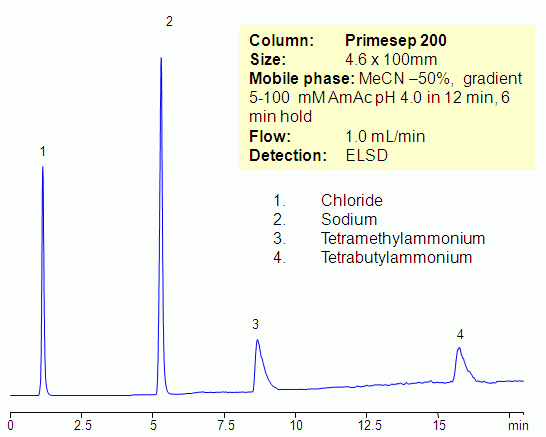
HPLC Separation of Sodium, Potassium Ions and Tetramethylammonium Chloride on Newcrom AH Column
September 8, 2020
HPLC Method for Analysis of Tetramethylammonium, Sodium, Potassium on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tetramethylammonium, Sodium, Potassium.
Tetramethylammonium chloride is a quaternary ammonium salt used widely as a reagent in industrial applications. It can be separated from sodium and potassium chlorides on a mixed-mode Newcrom AH column with a simple isocratic MS-compatible mobile phase of water, acetonitrile (ACN) and ammonium formate (AmFm) buffer.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | AmFm pH 3.0- 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | CAD (Corona) MS- compatible mobile phase |
| Class of Compounds | Hydrophilic, Metal, Ion, Quaternary ammonium salt |
| Analyzing Compounds | Tetramethylammonium, Sodium, Potassium |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Sodium
Tetramethylammonium

Separation of Sodium, Tetramethylammonium and Tetrabutylammonium on Primesep 200 Column
July 7, 2011
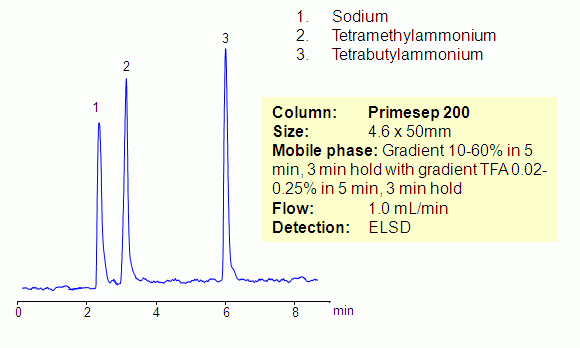
Hydrophilic and hydrophobic quaternary amines, along with sodium ion, were separated by mixed-mode chromatography on a Primesep 200 column. Mechanism of retention for sodium and tetramethylammonium ions is cation exchange, while the tetrabutylammonium ion is retained by combination of reversed-phase and cation-exchange mechanisms. All three compounds are not UV-active and monitoring is done by ELSD/CAD.
| Column | Primesep 200, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Ions, Hydrophilic, Ionizable, Quaternary amines |
| Analyzing Compounds | Sodium, Chloride, Tetramethylammonium, Tetrabutylammonium |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTetrabutylammonium
Tetramethylammonium

Separation of Quaternary Amines
September 11, 2003
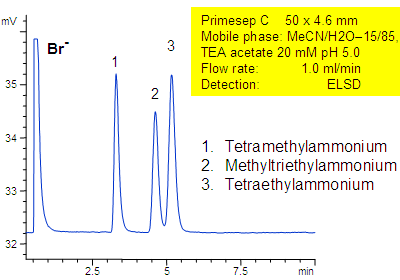
Primesep C separates a mixture of quaternary amines by a combination of cation exchange, complex formation, and hydrophic interactions. Methyltriethylammonium, tetraethylammonium, and tetramethylammonium cations as well as bromide counter ion are separated on a short 50 mm column. The separation uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and triethylamine acetate with evaporative light scattering detection (ELSD).
| Column | Primesep C, 4.6×50 mm |
| Mobile Phase | MeCN/H2O |
| Buffer | TEA acetate |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Quaternary Amines, Ionizable, Hormone |
| Analyzing Compounds | Methyltriethylammonium, tetraethylammonium, Tetramethylammonium |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethyltriethylammonium Bromide
Quaternary Amines
Tetraethylammonium
Tetramethylammonium