| CAS Number | 7758-98-7 |
|---|---|
| Molecular Formula | CuO4S |
| Molecular Weight | 159.600 |
| InChI Key | ARUVKPQLZAKDPS-UHFFFAOYSA-L |
| LogP | -1.03 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Copper (II) Sulfate
July 15, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Copper Sulfate check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

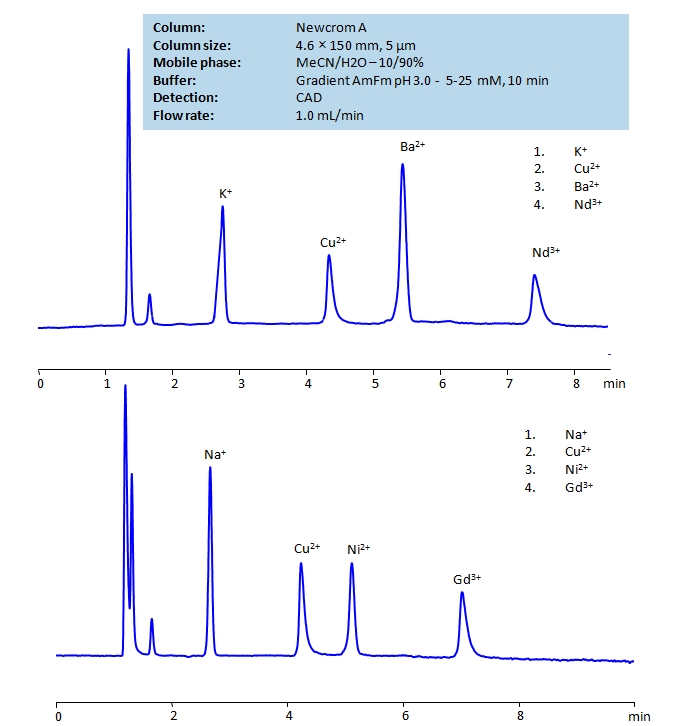
HPLC Determination of Ions on Newcrom A Column
March 24, 2020
HPLC Method for Sodium, Copper, Nickel, Gadolinium acetate, Barium, Neodymium, Copper Sulfate on Newcrom A by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Sodium, Copper, Nickel, Gadolinium acetate, Barium, Neodymium, Copper Sulfate.
Potassium ions, K+, are essential for cell function. They work as charge carriers inside animal cells to create the membrane. Imbalance of potassium can lead to debilitating health problems, but consumption of it through a diet can help regulate the negative effects of sodium on blood flow.
Copper ions, Cu2+, are known for their excellent electrical and thermal conductivity. Copper has been used since ancient times for tools, coins, and plumbing. These days, it is typically used in electrical wiring, construction, and industrial machinery. Timelessly, it plays a vital role in enzymes and red blood cell formation.
Barium ions, Ba2+, are typically used in quantum computing with trapped-ion computers, serving as a stable platform for qubits. It’s other uses include ceramics, medical imaging as a contrast agent in X-ray and CT scans, and in experimental physics for beta decay process.
Neodymium ions, Nd3+, have a variety of uses including glass, lasers, and promoting plant growth. Most notably, Neodymium magnets are the strongest permanent magnets known. They can lift a thousand times its own weight and snap together with enough force t break bones. The major drawback of Neodymium magnets is that they lose their magnetism at low temperatures.
Sodium ions, which have a chemical symbol of Na+, are a soft alkali and highly reactive metal. Sodium is found in abundance in everyday materials like table salt, sea water, and even the Earth’s crust. It is crucial for the body’s function and fluid balance.
Nickel ions, Ni2+, are considered the most common and stable oxidation state for nickel, which is a lustrous metal. In this state, it forms compounds easily with all common anions. Ni2+ is often found as green hexahydrate.
Gadolinium ions, Gd3+, have a variety of uses. Due to Gadolinium’s high neutron cross-section, it is effective in neutron radiography and in shielding of nuclear reactors. It is also used as an MRI contrasting agent, in experiments to study calcium channels, and fuel.
Sodium, Copper, Nickel, Gadolinium acetate, Barium, Neodymium, Copper Sulfate can be retained and analyzed using the Newcrom A stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with an ammonium formate buffer. Detection is performed using CAD.
| Column | Newcrom A, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 10% |
| Buffer | Gradient Ammonium formate pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds | Hydrophilic, Metal, Ion |
| Analyzing Compounds | Sodium, Copper, Nickel, Gadolinium acetate, Barium, Neodymium, Copper Sulfate |
Application Column
Newcrom A
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Copper
Copper Sulfate
Gadolinium acetate
Neodymium
Nickel
Sodium

HPLC UV Analysis of Copper Ions in Salt Mixture
July 3, 2013
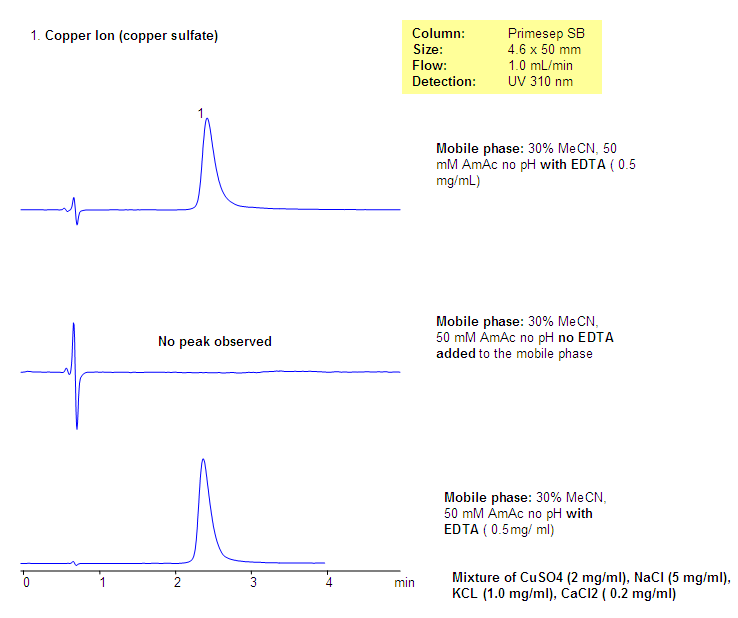
A unique approach for analysis of copper ions in a high salt content matrix was developed using EDTA as a visualization agent. Since copper ions can form colored complexes with EDTA, this property can be used for selective analysis of copper in the mixture with other cations, which do not form a colored complex with EDTA. Copper ion can be monitored by UV while other metals pass through the column undetected by UV. This method can be used to analyze copper ion at parts per million concentrations.
| Column | Primesep SB, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | AmAc, EDTA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 310 nm |
| Class of Compounds |
Ions |
| Analyzing Compounds | Copper |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCopper Sulfate