| CAS Number | 10605-21-7 |
|---|---|
| Molecular Formula | C9H9N3O2 |
| Molecular Weight | 191.191 |
| InChI Key | TWFZGCMQGLPBSX-UHFFFAOYSA-N |
| LogP | 1.52 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Carbendazim on Primesep 100 Column
August 2, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Carbendazim on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Carbendazim
Carbendazim is a fungicide widely used to control a variety of fungal diseases in crops.
arbendazim acts by inhibiting the development of fungi. It disrupts microtubule formation during mitosis, which prevents cell division and growth of the fungi.
Uses
Agricultural Applications:
Used on a variety of crops including fruits, vegetables, and cereals.
Controls a wide range of fungi, including those causing powdery mildew, leaf spots, and root rot.
Post-Harvest Treatment:
Used to treat fruits and vegetables after harvest to prevent spoilage during storage and transport.
Seed Treatment:
Applied to seeds before planting to protect against soil-borne fungal infections.
Carbendazim can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm.
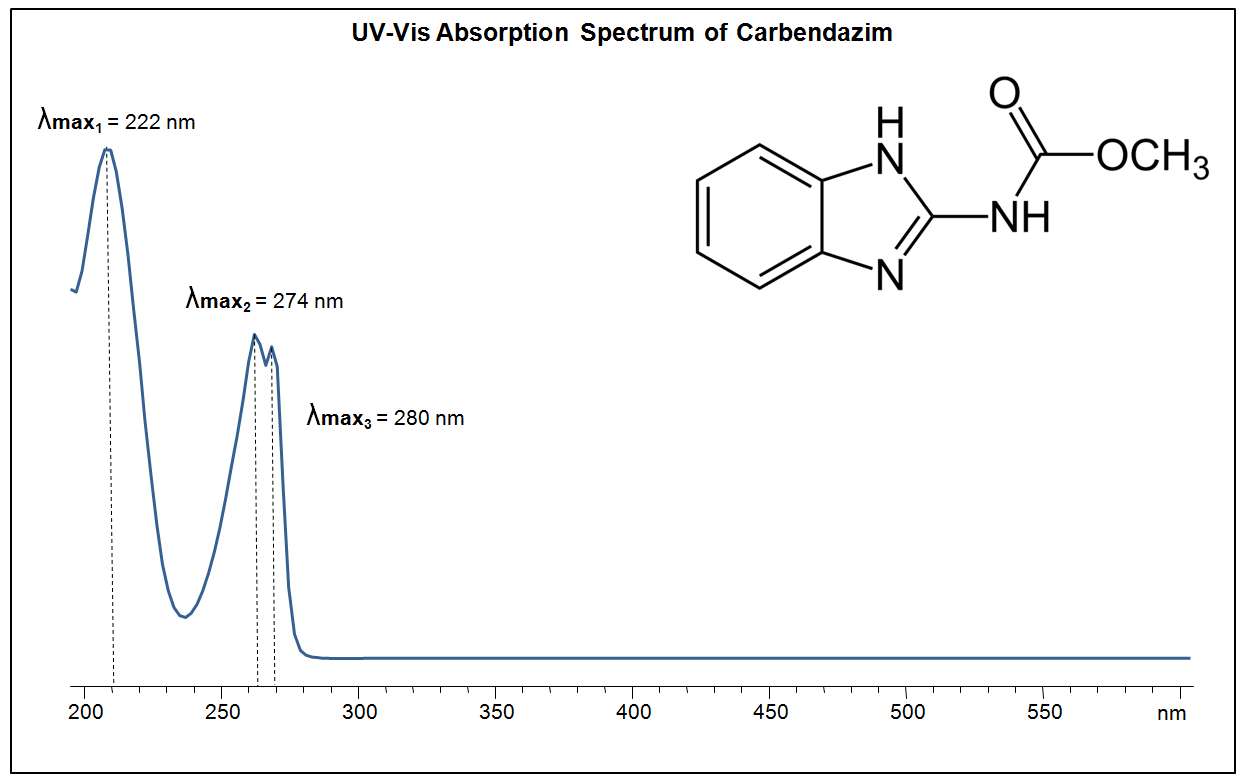
You can find detailed UV spectra of Carbendazim and information about its various lambda maxima by visiting the following link.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 70% |
| Buffer | H2SO4 -0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | 1.0 mg/ml in MP |
| Injection volume | 1 µl |
| LOD* | 40 ppb (200 nm) |
| Class of Compounds | Benzimidazoles |
| Analyzing Compounds | Carbendazim |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

UV-Vis Spectrum of Carbendazim
July 1, 2024
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
Carbendazim hydrobromide
Carbendazim hydrochloride

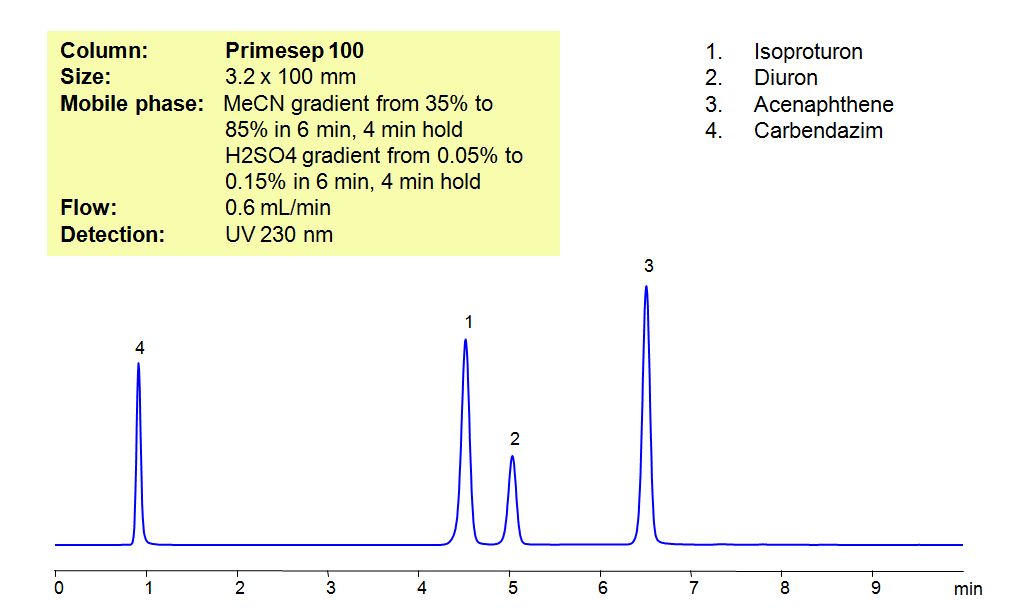
HPLC Method for Analysis of Pesticides: Isoproturon, Diuron, Acenaphthene, Carbendazim
July 11, 2017
Isoproturon is an agricultural herbicide with a tendency to adsorb to soils, which leads to it entering bodies of water. In that water, it is highly toxic for algae and oysters, while long term exposure to the pesticide affects the growth of fish. Diuron is a pre-emergent that is used for non-crop areas as well as with many agricultural crops. It has a low acute toxicity to mammals. It can cause irritation of nose and throat.if inhaled and irritation of eyes if comes in contact with the eyes. Acenaphthene is derived from coal tar and has many used such as dyes, pharmaceuticals, insecticides, fungicides, and plastics. It is harmful if ingested, inhaled or comes in contact with skin, and can cause irritations to skin, eyes, upper respiratory tract, and mucous membranes Carbendazim, also known as mercazole, is a broad-spectrum benzimidazole fungicide. It’s used mostly as a worm control agent in places like tennis courts and golf greens. Sometimes it is also used to control plant diseases in cereals and fruits. Primesep 100, a reverse-phase column, contains embedded acidic ionizable groups and can retain Isoproturon, Diuron, Acenaphthene, and Carbendazim. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
| Column | Primesep 100, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 35-85%, 6 min , 4 min hold |
| Buffer | Gradient H2SO4 – 0.05- 0.15%, 6 min, 4 min hold |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Herbicide, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Isoproturon, Diuron, Acenaphthene, Carbendazim |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCarbendazim
Diuron
Isoproturon

HPLC Separation of Bendazoles on Primesep P Column
August 22, 2008
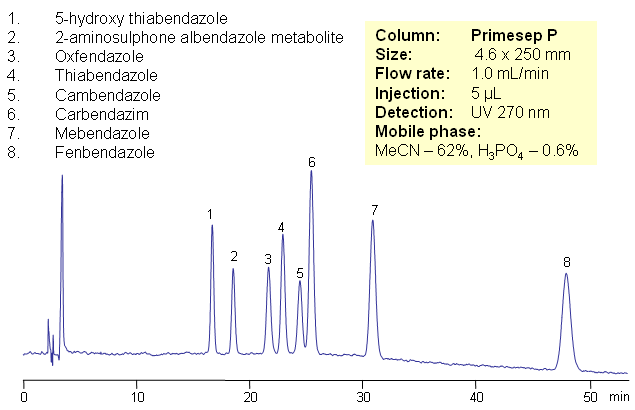
Eight bendazoles are separated on Primesep P HPLC column by combination of reverse phase and ion-exchange mechanisms. Primesep P column can be used when mixed-mode mechanisms, including pi-pi interactions, can benefit separation. HPLC column and method is compatible with UV detection technique.
Application Column
Primesep P
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options5-Hydroxy Thiabendazole
Albendazole
Cambendazole
Carbendazim
Fenbendazole
Mebendazole
Oxfendazole
Thiabendazole