| CAS Number | 330-54-1 |
|---|---|
| Molecular Formula | C9H10Cl2N2O |
| Molecular Weight | 233.090 |
| InChI Key | XMTQQYYKAHVGBJ-UHFFFAOYSA-N |
| LogP | 2.68 |
| Synonyms |
|
Applications:
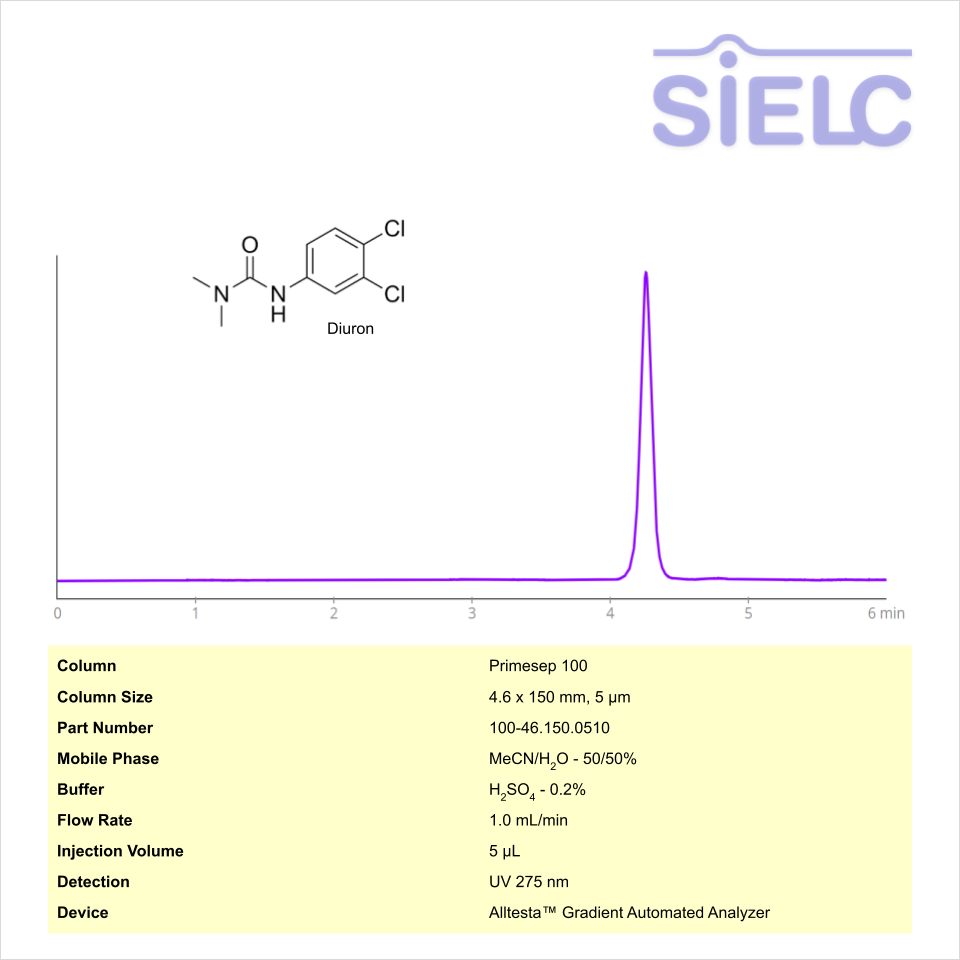
HPLC Method for Analysis of Diuron on Primesep 100 Column on Alltesta™
November 19, 2025
HPLC Method for Diuron on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Diuron
Diuron, also known as DCMU (3-(3,4-dichlorophenyl)-1,1-dimethylurea), is an algicide and herbicide with the chemical formula C9H10Cl2N2O. It works through inhibiting photosynthesis primarily broadleaf weeds such as crabgrass, foxtail, and pigweed. It is toxic when ingested and there are studies being conducted to test for whether or not it is a carcinogen. You can find detailed UV spectra of Diuron and information about its various lambda maxima by visiting the following link.
Diuron can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with phosphoric acid as a buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 50% |
| Buffer | Sulfuric Acid |
| Flow Rate | 1.0 mL/min |
| Detection | UV 275 nm |
| Class of Compounds | Herbicide |
| Analyzing Compounds | Diuron |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

UV-Vis Spectrum of Diuron
July 12, 2024
Access the UV-Vis Spectrum SIELC Library
UV-Vis Spectrum of Diuron. Absorption Maxima: 212 nm, 250 nm, 287nm.
If you are looking for optimized HPLC method to analyze Diuron check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

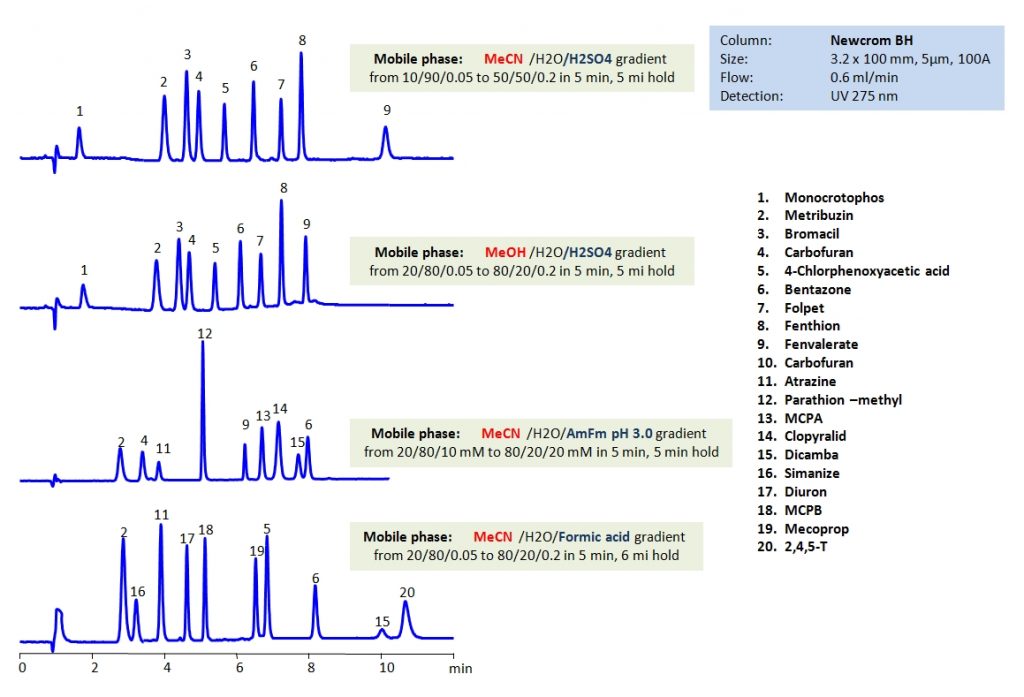
HPLC Separation of Pesticides, Herbicides, Fungicides, and Insecticides on Newcrom BH Column
May 22, 2020
HPLC Method for Mecoprop, MCPB, Diuron, Simazine, Dicamba, Clopyralid, MCPA, Atrazine, Carbofuran, Fenvalerate, Folpet, Bentazone-sodium, 2,4,5-Trichlorophenoxyacetic acid, 4-CPA, Methyl parathion, Metribuzin, Monocrotophos on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Mecoprop, MCPB, Diuron, Simazine, Dicamba, Clopyralid, MCPA, Atrazine, Carbofuran, Fenvalerate, Folpet, Bentazone-sodium, 2,4,5-Trichlorophenoxyacetic acid, 4-CPA, Methyl parathion, Metribuzin, Monocrotophos.
Herbicides are used to control unwanted plants, they are also known as weedkillers. Insecticides are used to kill insects. Fungicides are used to kill parasitic fungi. Pesticide is a more generic term that includes herbicides, fungicides and insecticides in its definition. All are heavily used in agriculture.
By using HPLC, many different pesticides can be separated and their retention characteristics controlled using the Newcrom BH mixed-mode column. It can be used with different organic media such as acetonitrile (ACN) or methanol (MeOH). By varying the concentration of organic modifier and using different buffers like sulfuric acid (H2SO4), ammonium formate (AmFm), or formic acid, separation of desired pesticides can be achieved.
| Column | Newcrom BH, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN, MeOH |
| Buffer | H2SO4, Formic acid, AmFm pH 3.0 |
| Flow Rate | 0.6 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Pesticides, Herbicides, Fungicides, Insecticides |
| Analyzing Compounds | Mecoprop, MCPB, Diuron, Simazine, Dicamba, Clopyralid, MCPA, Atrazine, Carbofuran, Fenvalerate, Folpet, Bentazone-sodium, 2,4,5-Trichlorophenoxyacetic acid, 4-CPA, Methyl parathion, Metribuzin, Monocrotophos |
Application Column
Newcrom BH
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
4-CPA
Atrazine
Bentazone-sodium
Carbofuran
Clopyralid
Dicamba
Diuron
Fenvalerate
Folpet
MCPA
MCPB
Mecoprop
Methyl parathion
Metribuzin
Monocrotophos
Simazine

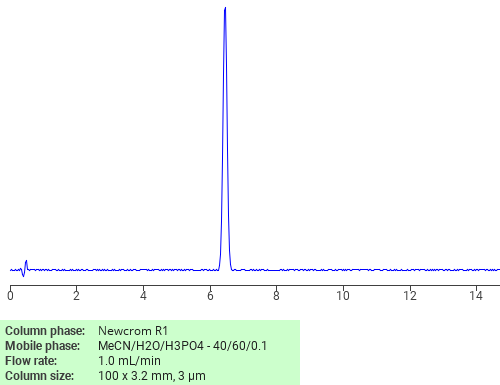
Separation of Diuron on Newcrom R1 HPLC column
February 16, 2018
Diuron can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
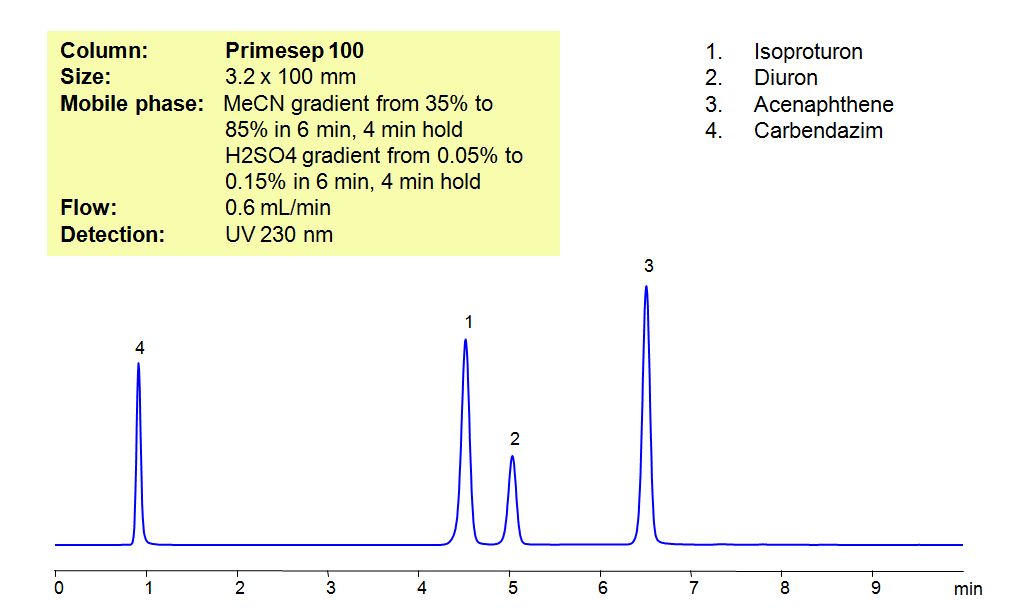
Select optionsHPLC Method for Analysis of Pesticides: Isoproturon, Diuron, Acenaphthene, Carbendazim
July 11, 2017
Isoproturon is an agricultural herbicide with a tendency to adsorb to soils, which leads to it entering bodies of water. In that water, it is highly toxic for algae and oysters, while long term exposure to the pesticide affects the growth of fish. Diuron is a pre-emergent that is used for non-crop areas as well as with many agricultural crops. It has a low acute toxicity to mammals. It can cause irritation of nose and throat.if inhaled and irritation of eyes if comes in contact with the eyes. Acenaphthene is derived from coal tar and has many used such as dyes, pharmaceuticals, insecticides, fungicides, and plastics. It is harmful if ingested, inhaled or comes in contact with skin, and can cause irritations to skin, eyes, upper respiratory tract, and mucous membranes Carbendazim, also known as mercazole, is a broad-spectrum benzimidazole fungicide. It’s used mostly as a worm control agent in places like tennis courts and golf greens. Sometimes it is also used to control plant diseases in cereals and fruits. Primesep 100, a reverse-phase column, contains embedded acidic ionizable groups and can retain Isoproturon, Diuron, Acenaphthene, and Carbendazim. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
| Column | Primesep 100, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 35-85%, 6 min , 4 min hold |
| Buffer | Gradient H2SO4 – 0.05- 0.15%, 6 min, 4 min hold |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Herbicide, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Isoproturon, Diuron, Acenaphthene, Carbendazim |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCarbendazim
Diuron
Isoproturon