| CAS Number | 201-469-6 |
|---|---|
| Molecular Formula | C12H10 |
| Molecular Weight | 154.212 |
| InChI Key | CWRYPZZKDGJXCA-UHFFFAOYSA-N |
| LogP | 3.9 |
| Synonyms |
|
Applications:
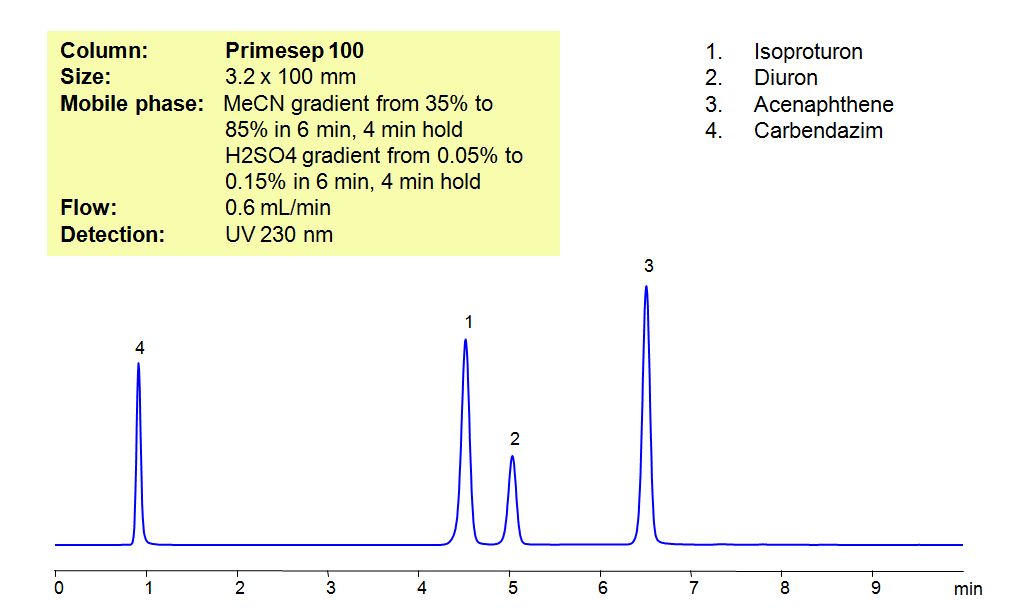
HPLC Method for Analysis of Pesticides: Isoproturon, Diuron, Acenaphthene, Carbendazim
July 11, 2017
Isoproturon is an agricultural herbicide with a tendency to adsorb to soils, which leads to it entering bodies of water. In that water, it is highly toxic for algae and oysters, while long term exposure to the pesticide affects the growth of fish. Diuron is a pre-emergent that is used for non-crop areas as well as with many agricultural crops. It has a low acute toxicity to mammals. It can cause irritation of nose and throat.if inhaled and irritation of eyes if comes in contact with the eyes. Acenaphthene is derived from coal tar and has many used such as dyes, pharmaceuticals, insecticides, fungicides, and plastics. It is harmful if ingested, inhaled or comes in contact with skin, and can cause irritations to skin, eyes, upper respiratory tract, and mucous membranes Carbendazim, also known as mercazole, is a broad-spectrum benzimidazole fungicide. It’s used mostly as a worm control agent in places like tennis courts and golf greens. Sometimes it is also used to control plant diseases in cereals and fruits. Primesep 100, a reverse-phase column, contains embedded acidic ionizable groups and can retain Isoproturon, Diuron, Acenaphthene, and Carbendazim. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
| Column | Primesep 100, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 35-85%, 6 min , 4 min hold |
| Buffer | Gradient H2SO4 – 0.05- 0.15%, 6 min, 4 min hold |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Herbicide, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Isoproturon, Diuron, Acenaphthene, Carbendazim |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCarbendazim
Diuron
Isoproturon

HPLC Separation of Aromatic Compounds (PAH) on Mixed-Mode and Reverse Phase Columns
October 4, 2007
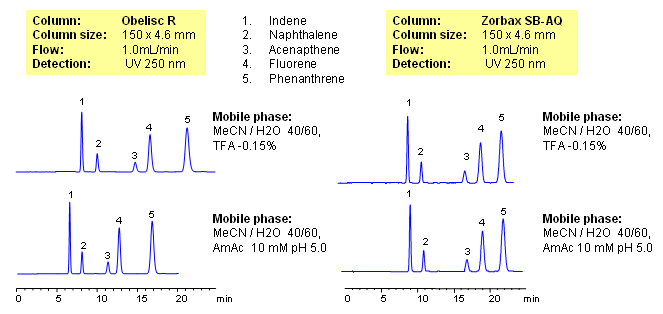
Aromatic hydrocarbons are hydrophobic compounds which are well retained on any reverse column. Retention time is adjusted by the amount of ACN. Change in buffer concentration or buffer pH does not affect retention time. Method on Obelisc mixed-mode column shows retention and separation of PAHs by reverse phase mechanism. Change of pH is changing conformation and ionization of stationary phase on Obelisc R column, making it more or less hydrophobic. This changes interaction on the column and selectivity of separation. This method can be used for analysis of hydrophobic compounds and isomers by reverse phase mechanism, when fine tuning is required to achieve desired degree of separation.
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsFluorene
Indene
Naphthalene
Phenanthrene