| CAS Number | 99-50-3 |
|---|---|
| Molecular Formula | C7H6O4 |
| Molecular Weight | 154.122 |
| InChI Key | YQUVCSBJEUQKSH-UHFFFAOYSA-N |
| LogP | 0.86 |
| Synonyms |
|
Applications:
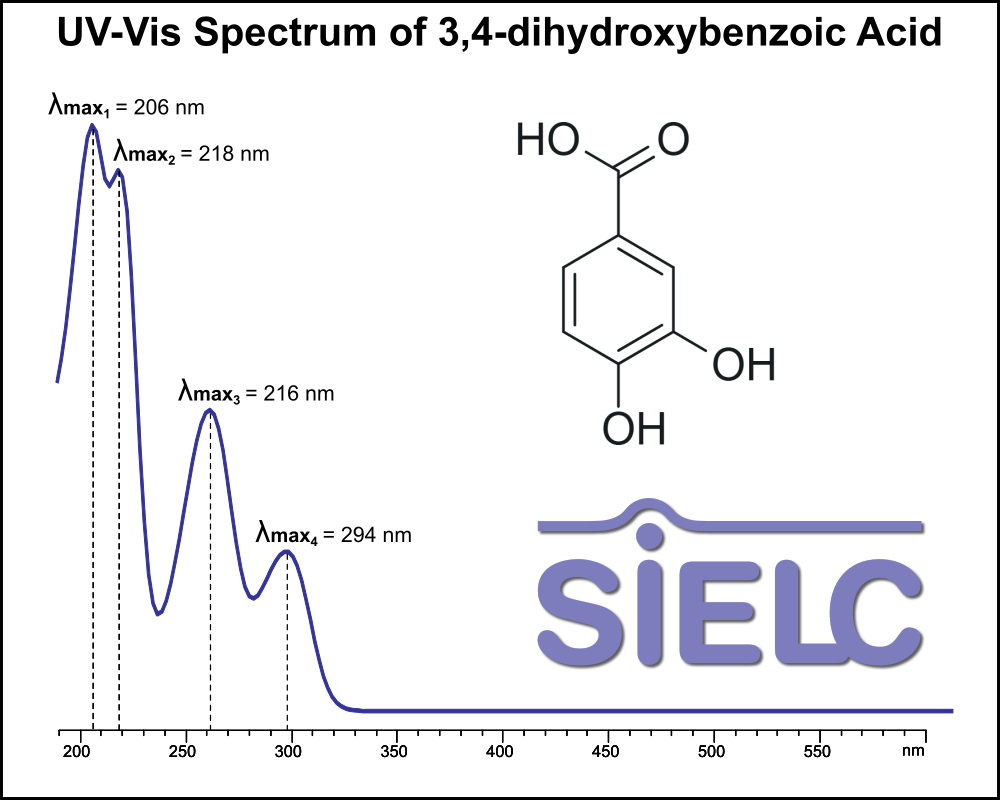
UV-Vis Spectrum of 3,4-dihydroxybenzoic Acid
August 1, 2025
If you are looking for optimized HPLC method to analyze 3,4-Dihydroxybenzoic Acid check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Separation of Phenolic Acids
March 11, 2021
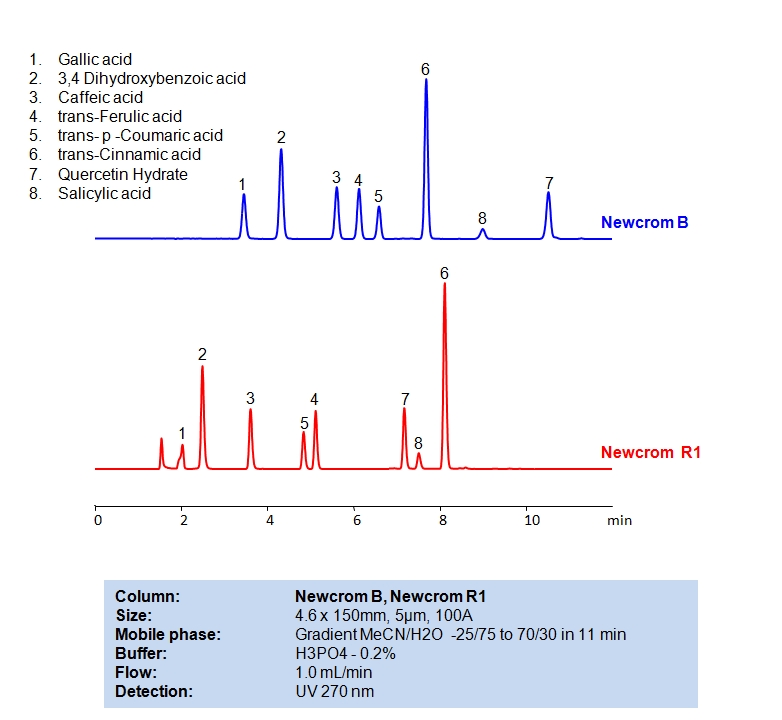
HPLC Method for Gallic acid, 3,4-Dihydroxybenzoic Acid, Caffeic acid, Ferulic acid, p-coumaric acid, Cinnamic acid, Salicylic acid, Quercetin on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Gallic acid, 3,4-Dihydroxybenzoic Acid, Caffeic acid, Ferulic acid, p-coumaric acid, Cinnamic acid, Salicylic acid, Quercetin.
Gallic acid is a naturally-occurring phenolic acid that was a key ingredient in European iron gall ink from its first discovery in the 12th century until the 19th century. In modern times, it’s heavy metal salt, Bismuth subgallate, known by its trade name Devrom, is known for its effects at deodorizing flatulence and stools. It has the chemical formula C7H6O5.
3,4 Dihydroxybenzoic acid, also known as Protocatechuic acid (PCA), is a dihydroxybenzoic acid with the chemical formula C7H6O4. It occurs naturally in stem bark of Boswellia dalzielii, leaves of Diospyros melanoxylon, Açai oil, and roselle. Commercially , it is produced of vanillin. You can find detailed UV spectra of 3,4 Dihydroxybenzoic acid and information about its various lambda maxima by visiting the following link.
Caffeic acid is an organic compound with the formula (HO)2C6H3CH=CHCO2H. It plays a key role in scavenging reactive oxygen species (ROS) that are generated during energy metabolism. Caffeic acid is an active ingredient in caffenol, which is a photographic developer made from instant coffee. Pharmacologically, it is used as an antioxidant in vitro and also in vivo. It also shows immunomodulatory and anti-inflammatory activity.
trans-Ferulic acid is a hydroxycinnamic acid derivative with the chemical formula C10H10O4. It is classified as a phenolic phytochemical. Naturally, it can be found in a large number of vegetables, grain, and other plants. It is also released while sweetcorn is being cooked. Salts and esters derived from ferulic acid are called ferulates.
trans-p-Coumaric acid is a major phenolic acid with the chemical formula C9H8O3. It can be found in a wide variety of graminaceous plants. p-Coumaric acid is a natural metabolite contained in many edible plants and its antioxidant activities in reducing oxidative stress and inflammatory reactions have been demonstrated in various experiments.
trans-Cinnamic acid and benzoic acid are two of the major phenolic acids and autotoxins. Cinnamic acid occurs naturally in all green plants, showing significant antimicrobial activity against both bacteria and fungi as well as antioxidant activity. It has the chemical formula C9H8O2.
Quercetin Hydrate is a naturally occurring flavonol, or flavonoid, the yellowish antioxidant pigment found in skins of red grapes, apples, berries, onions, tomatoes, and buckwheat tea. In addition to functioning as a flavonoid, quercetin is also a phytoestrogen. It has the chemical formula C15H10O7.
Salicylic acid (SA) is an o-hydroxybenzoic acid, synthesized by both plants and microorganisms. SA acts as a critical plant hormone regulating various processes, including growth and development, flowering, thermogenesis, ion uptake, stomatal movement, photosynthesis, and plant immunity. SA also has medical applications as a topical treatment for a wide variety of skin conditions, including warts, dandruff, and acne.
Gallic acid, 3,4-Dihydroxybenzoic Acid, Caffeic acid, Ferulic acid, p-coumaric acid, Cinnamic acid, Salicylic acid, Quercetin can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a phosphoric acid buffer. Detection is performed using UV.
| Column | Newcrom B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN |
| Buffer | H3PO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | 270 nm |
| Column | Newcrom R1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN |
| Buffer | H3PO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | 270 nm |
| Class of Compounds | Acid |
| Analyzing Compounds | Gallic acid, 3,4-Dihydroxybenzoic Acid, Caffeic acid, Ferulic acid, p-coumaric acid, Cinnamic acid, Salicylic acid, Quercetin |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Newcrom R1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Caffeic acid
Cinnamic acid
Ferulic acid
Gallic acid
Quercetin
Salicylic acid
p-coumaric acid

HPLC Separation of Dihydroxybenzoic Acids on Newcrom B Column
April 24, 2020
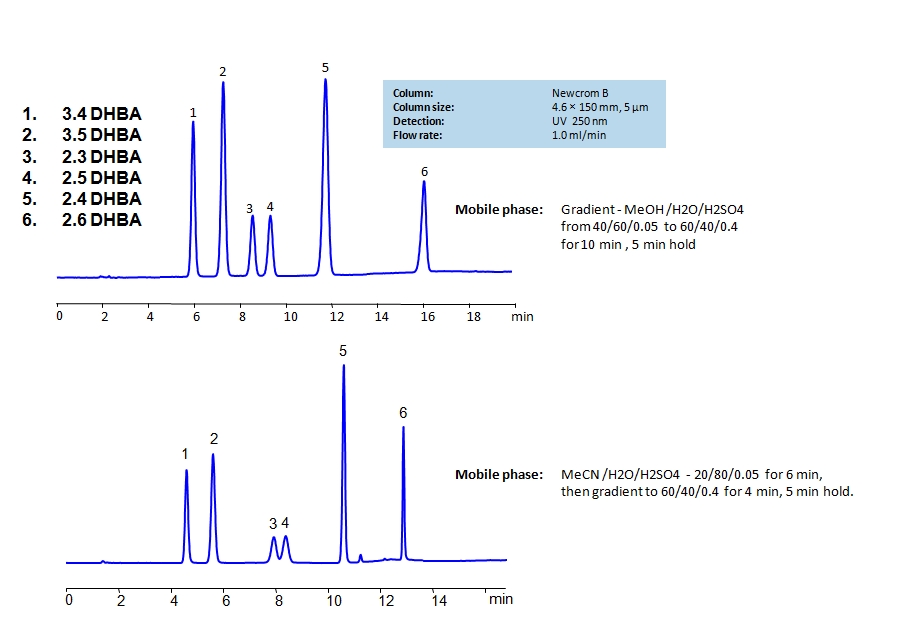
HPLC Method for 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid.
Dihydroxybenzoic acids are aromatic compounds consisting of a phenolic ring and a carboxylic acid. They all have the same chemical formula C7H6O4.
The six main compounds are structurally similar and are difficult to separate in reverse-phase HPLC. The can be separated by using a mixed-mode Newcrom B column with the mobile phase having either methanol (MeOH) or acetonitrile (ACN) as an organic modifier having different retention characteristics. Using the gradient of organic modifier, water and sulfuric acid (H2SO4) as buffer, dihydroxybenzoic acids can be separated and UV detected at 250nm.
You can find detailed UV spectra of 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid and information about its various lambda maxima by visiting the following links for 3.4 DHBA, 3.5 DHBA, 2.4 DHBA, and 2.5 DHBA.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
2,6-Dihydroxybenzoic acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

HPLC Separation of Dihydroxybenzoic Acid
November 21, 2010
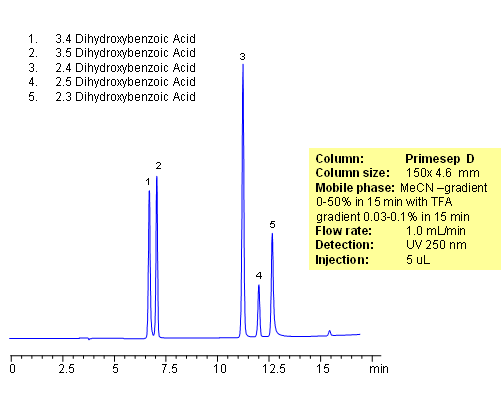
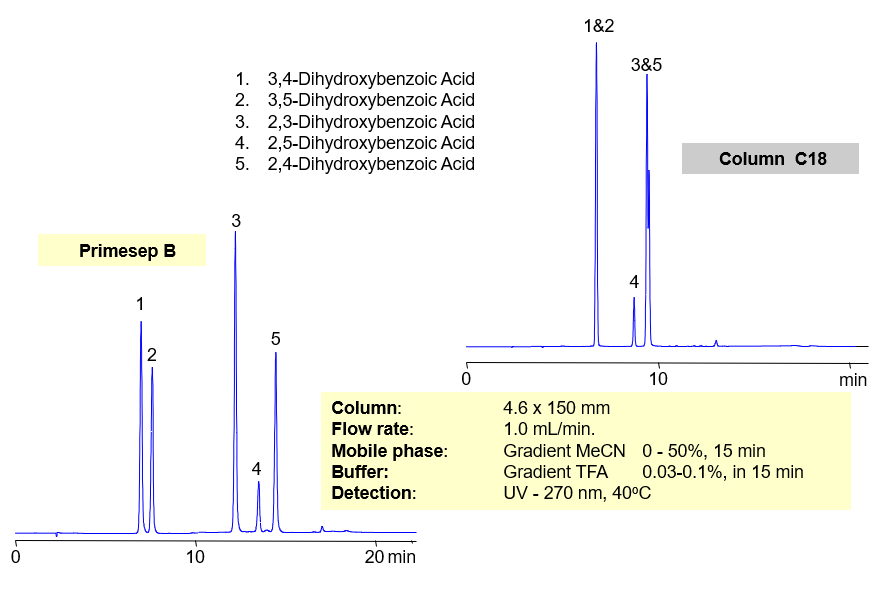
Separation of hydrophilic isomers is very difficult when only one mechanism of retention, like reversed-phase, is available. Isomers have very similar hydrophobic properties. In case of mixed-mode chromatography, small difference in hydrophobic and ionic properties allows to separate isomers. Isomers of dihydroxybenzoic acids are separated on a Primesep D column with good selectivity and peak shape. This method can be used for separation of other hydrophilic acidic compounds as well as hydrophobic basic compound.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

Mixed-Mode Separation of Dihydroxybenzoic Acids
October 12, 2005
Primesep B offers better selectivity over traditional, reversed-phase C18 columns for separating regioisomers of aromatic dihydroxybenzoic acids (2,3-dihydroxybenzoic, 2,4-dihydroxybenzoic, 2,5-dihydroxybenzoic 3,4-dihydroxybenzoic, 3,5-dihydroxybenzoic acids) Primesep B combines reversed-phase and anion-exchange mechanism with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA).
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid
Carboxylic Acids