| CAS Number | 89-86-1 |
|---|---|
| Molecular Formula | C7H6O4 |
| Molecular Weight | 154.122 |
| InChI Key | UIAFKZKHHVMJGS-UHFFFAOYSA-N |
| LogP | 1.63 |
| Synonyms |
|
Applications:
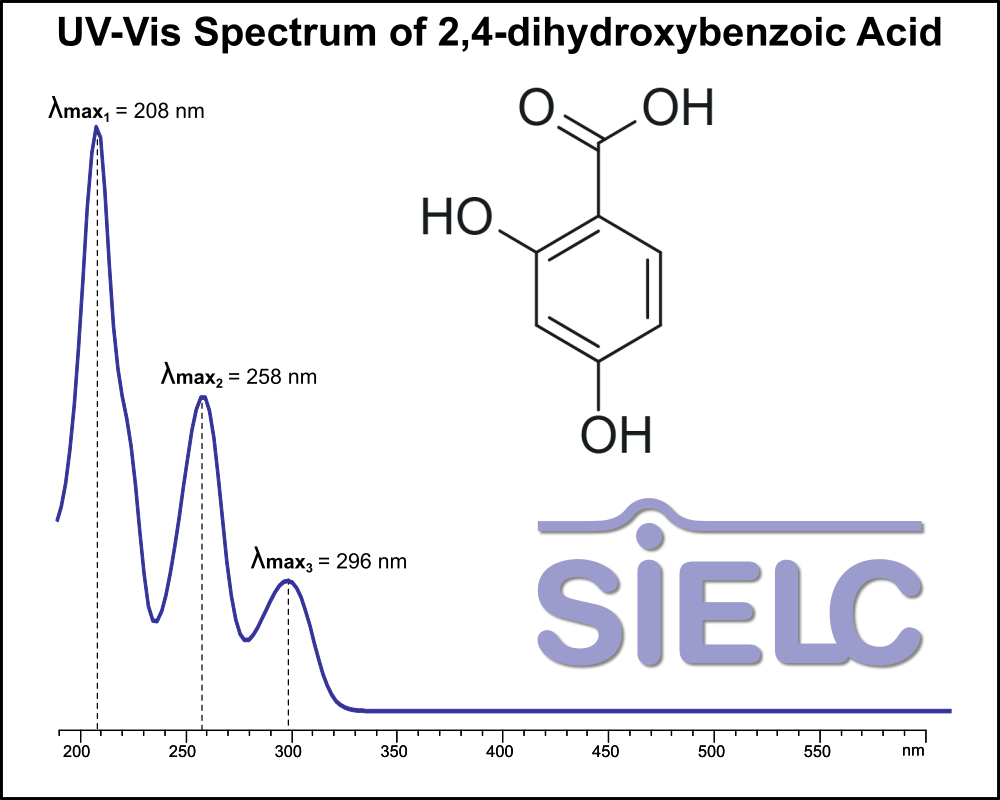
UV-Vis Spectrum of 2,4-dihydroxybenzoic Acid
August 1, 2025
If you are looking for optimized HPLC method to analyze 2,4-Dihydroxybenzoic Acid check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

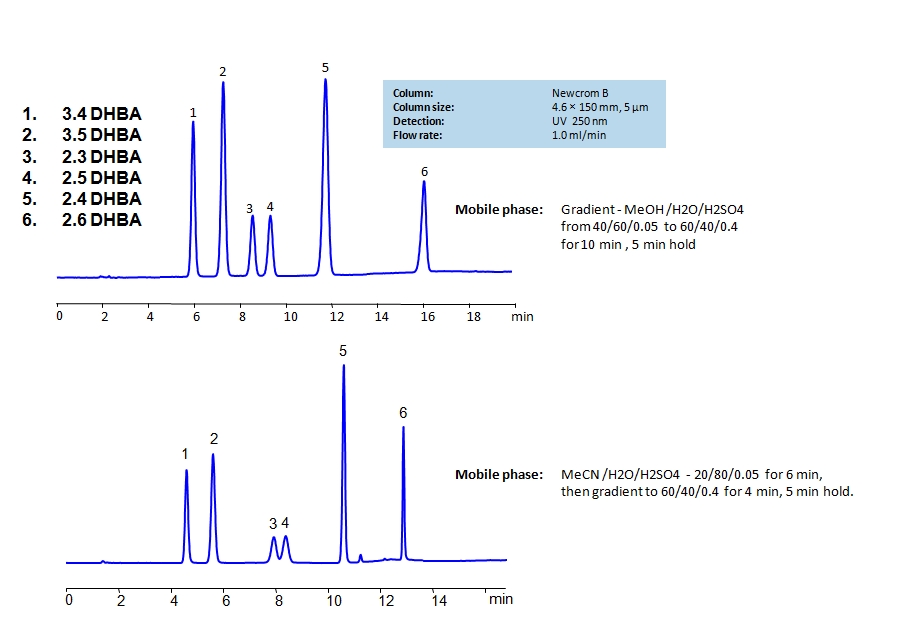
HPLC Separation of Dihydroxybenzoic Acids on Newcrom B Column
April 24, 2020
HPLC Method for 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid.
Dihydroxybenzoic acids are aromatic compounds consisting of a phenolic ring and a carboxylic acid. They all have the same chemical formula C7H6O4.
The six main compounds are structurally similar and are difficult to separate in reverse-phase HPLC. The can be separated by using a mixed-mode Newcrom B column with the mobile phase having either methanol (MeOH) or acetonitrile (ACN) as an organic modifier having different retention characteristics. Using the gradient of organic modifier, water and sulfuric acid (H2SO4) as buffer, dihydroxybenzoic acids can be separated and UV detected at 250nm.
You can find detailed UV spectra of 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid and information about its various lambda maxima by visiting the following links for 3.4 DHBA, 3.5 DHBA, 2.4 DHBA, and 2.5 DHBA.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 2,3-Dihydroxybenzoic Acid, 2,4-Dihydroxybenzoic Acid, 2,5-Dihydroxybenzoic Acid, 3,4-Dihydroxybenzoic Acid, 3,5-Dihydroxybenzoic Acid, 2,6-Dihydroxybenzoic acid |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
2,6-Dihydroxybenzoic acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

HPLC separation of Dihydroxybenzoic acid in Hydrogen-Bonding mode on SHARC 1 HPLC column
June 18, 2012
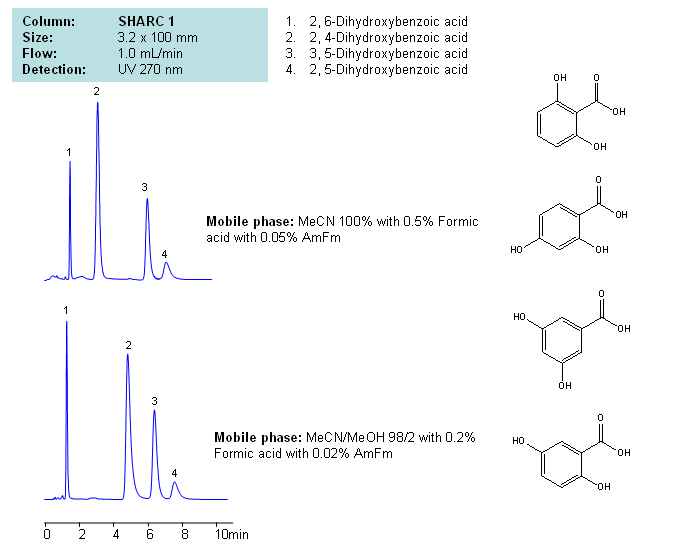
Application Notes: Dihydroxybenzoic acids are polar acidic compounds containing two hydroxyl groups as well as a carboxylic acid. Isomers of dihydroxybenzoic acids are very polar with varying degrees of acidity. While the isomers can be analyzed by mixed-mode chromatography on Primesep D column, the presence of hydroxyls and carboxylic acid fragments also make these compounds good candidates for using the new hydrogen bonding columns for analysis. Since all these compounds have the same amount of hydroxyls they are separated based on accessibility of this hydroxyl group. Retention time can be adjusted by the amount of ACN, MeOh and concentration of additives (formic acid, ammonium formate, triethylamine, etc.). Our method is fully compatible with LC/MS and prep chromatography and can be used for other hydroxy and carboxylic acids containing compounds..
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A. To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: 2,6-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid
Detection Technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 2,6-dihydroxybenzoic acid, 2,4-dihydroxybenzoic acid, 3,5-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid |
Application Column
SHARC 1
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
2,5-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

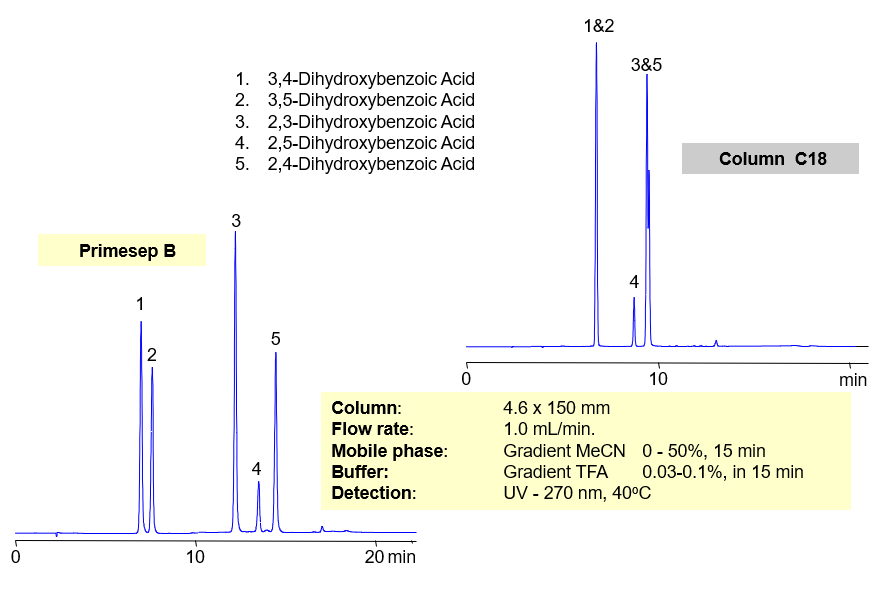
HPLC Separation of Dihydroxybenzoic Acid
November 21, 2010
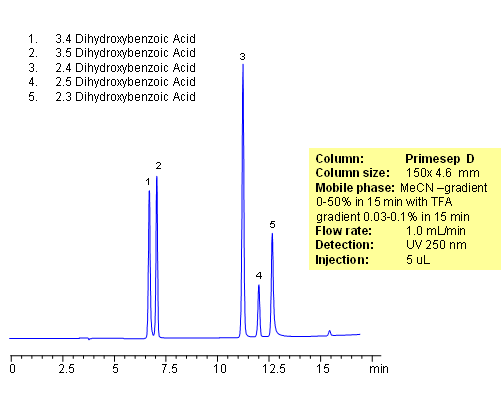
Separation of hydrophilic isomers is very difficult when only one mechanism of retention, like reversed-phase, is available. Isomers have very similar hydrophobic properties. In case of mixed-mode chromatography, small difference in hydrophobic and ionic properties allows to separate isomers. Isomers of dihydroxybenzoic acids are separated on a Primesep D column with good selectivity and peak shape. This method can be used for separation of other hydrophilic acidic compounds as well as hydrophobic basic compound.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

Mixed-Mode Separation of Dihydroxybenzoic Acids
October 12, 2005
Primesep B offers better selectivity over traditional, reversed-phase C18 columns for separating regioisomers of aromatic dihydroxybenzoic acids (2,3-dihydroxybenzoic, 2,4-dihydroxybenzoic, 2,5-dihydroxybenzoic 3,4-dihydroxybenzoic, 3,5-dihydroxybenzoic acids) Primesep B combines reversed-phase and anion-exchange mechanism with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA).
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid
Carboxylic Acids