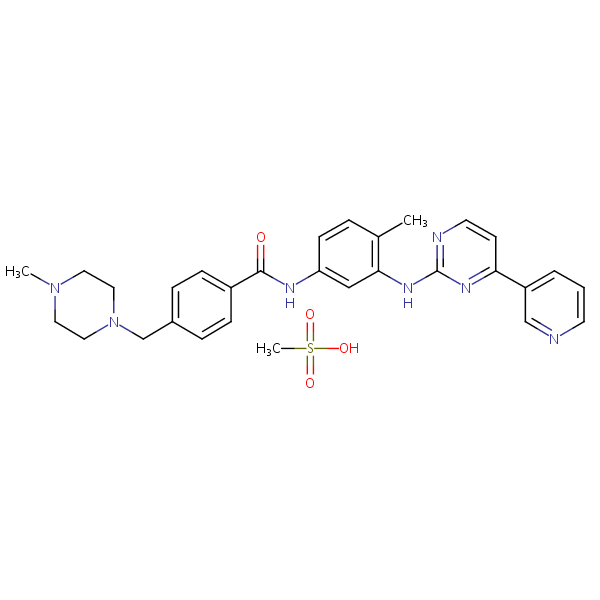
| CAS Number | 220127-57-1 |
|---|---|
| Molecular Formula | C30H35N7O4S |
| Molecular Weight | 589.720 |
| InChI Key | YLMAHDNUQAMNNX-UHFFFAOYSA-N |
| LogP | 2.84 |
| Synonyms |
|
Applications:
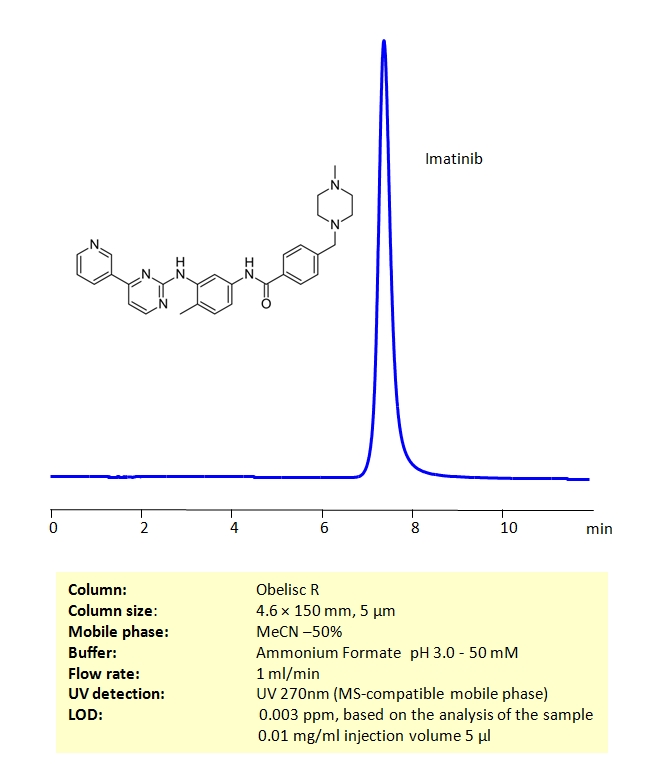
Determination Imatinib Mesylate on Obelisc R Column
February 10, 2021
HPLC Method for Imatinib mesylate on Obelisc R by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Imatinib mesylate.
Imatinib mesylate is a classified as a signal transduction inhibitor with the chemical formula C29H31N7O. It is an oral targeted therapy medication that is effective in treating chronic myeloid leukemia and metastatic gastrointestinal stromal tumors. Commercial versions of the drug were a topic of controversy when cancer specialists published a letter condemning the high costs of cancer treatments.
Imatinib mesylate can be retained on an Obelisc R column, which has both positive and negative ion-pairs embedded in the stationary phase, allowing for the fine tuning and separation of a wide range of compounds with different ionic properties. Imatinib mesylate can be determined isocratically using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Formate (AmFm) buffer and detected by UV at 270nm, ELSD, CAD or LC/MS.
| Column | Obelisc R, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN -50% |
| Buffer | Ammonium Formate pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270nm |
| Class of Compounds |
Drug |
| Analyzing Compounds | Imatinib mesylate |
Application Column
Obelisc R
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

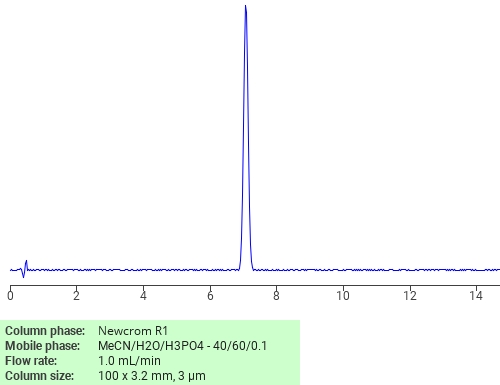
Separation of Imatinib mesylate on Newcrom R1 HPLC column
May 16, 2018
Imatinib mesylate can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options