HPLC Method for Imatinib mesylate on Obelisc R by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Imatinib mesylate.
Imatinib mesylate is a classified as a signal transduction inhibitor with the chemical formula C29H31N7O. It is an oral targeted therapy medication that is effective in treating chronic myeloid leukemia and metastatic gastrointestinal stromal tumors. Commercial versions of the drug were a topic of controversy when cancer specialists published a letter condemning the high costs of cancer treatments.
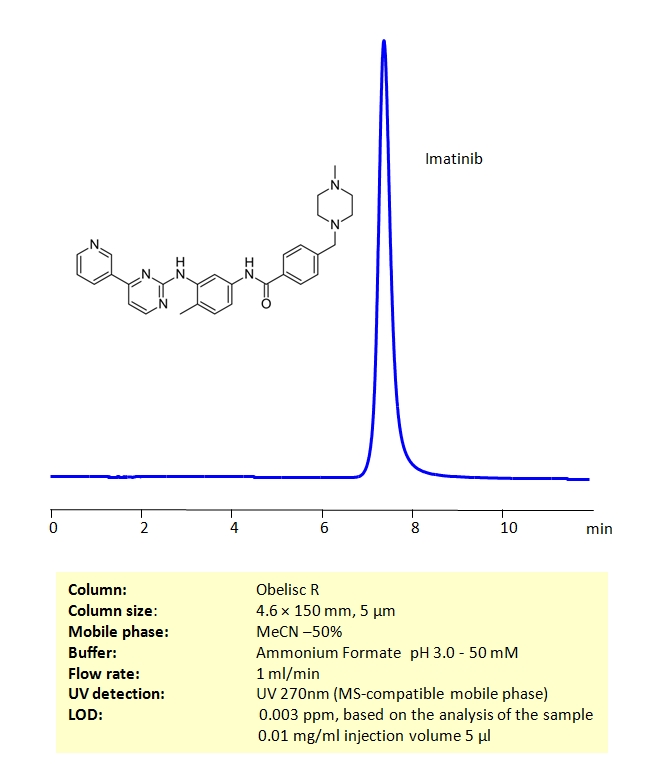
Imatinib mesylate can be retained on an Obelisc R column, which has both positive and negative ion-pairs embedded in the stationary phase, allowing for the fine tuning and separation of a wide range of compounds with different ionic properties. Imatinib mesylate can be determined isocratically using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Formate (AmFm) buffer and detected by UV at 270nm, ELSD, CAD or LC/MS.
| Column | Obelisc R, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN -50% |
| Buffer | Ammonium Formate pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270nm |
| Class of Compounds |
Drug |
| Analyzing Compounds | Imatinib mesylate |
Application Column
Obelisc R
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended