| CAS Number | 114873-37-9 |
|---|---|
| Molecular Formula | C14H26N4O6 |
| Molecular Weight | 346.384 |
| InChI Key | HHLZCENAOIROSL-UHFFFAOYSA-N |
| LogP | -8.4 |
| Synonyms |
|
Applications:
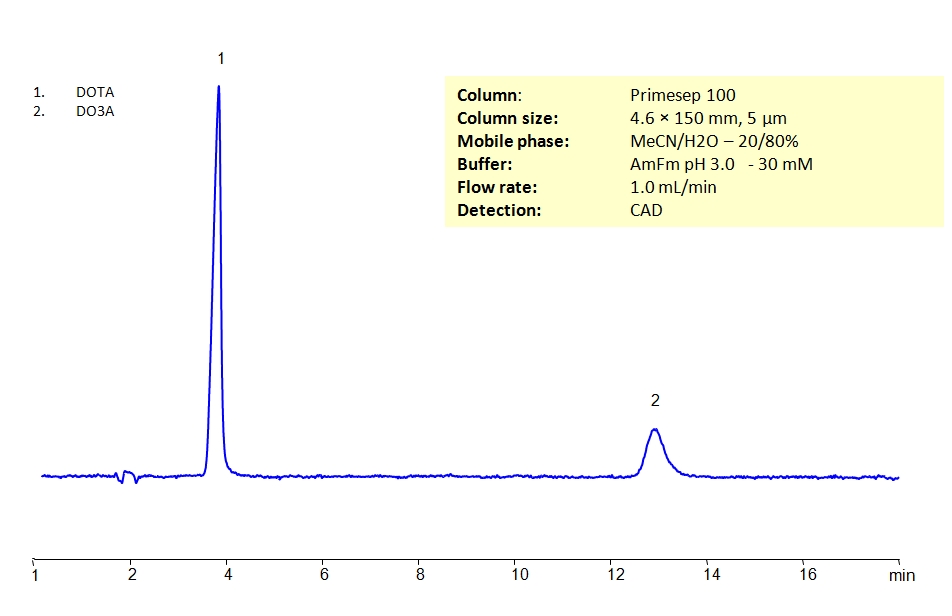
HPLC Separation of DOTA and DO3A MS- compatible mobile phase
September 25, 2019
HPLC Method for Tetraxetan (DOTA), DO3A on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tetraxetan (DOTA), DO3A
DOTA is a macrocyclic cheating agent with the chemical formula C16H28N4O8. DOTA is actually an acronym for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid. It is used to bind metal ions, partially due to it’s stability. It is often employed in biomedical applications including but not limited to MRI contrast agents and targeted cancer therapy.
DO3A is a derivative of DOTA with the chemical formula C₁₄H₂₃N₄O₆Na₃.
Tetraxetan (DOTA), DO3A can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with an ammonium formate buffer. Detection is performed using CAD.
Condition
Column Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended Mobile Phase MeCN/H2O – 20/80% Buffer AmFm pH 3.0- 30 mM Flow Rate 1.0 ml/min Detection CAD (Corona) MS- compatible mobile phase
Description
Class of Compounds
Acid, Hydrophilic, Ionizable, Carboxylic acid, Carbocyclic. Analyzing Compounds Tetraxetan (DOTA), DO3A
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Tetraxetan (DOTA)

HPLC Separation of DOTA and DO3A on Primesep 100 Column
September 25, 2019
HPLC Method for DO3A, Tetraxetan (DOTA) on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of DO3A, Tetraxetan (DOTA)
DOTA is a macrocyclic cheating agent with the chemical formula C16H28N4O8. DOTA is actually an acronym for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid. It is used to bind metal ions, partially due to it’s stability. It is often employed in biomedical applications including but not limited to MRI contrast agents and targeted cancer therapy.
DO3A is a derivative of DOTA with the chemical formula C₁₄H₂₃N₄O₆Na₃.
DO3A, Tetraxetan (DOTA) can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with an ammonium formate buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H2SO4 – 0.1 % |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable, Carboxylic acid, Carbocyclic. |
| Analyzing Compounds | DO3A, Tetraxetan (DOTA) |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Tetraxetan (DOTA)

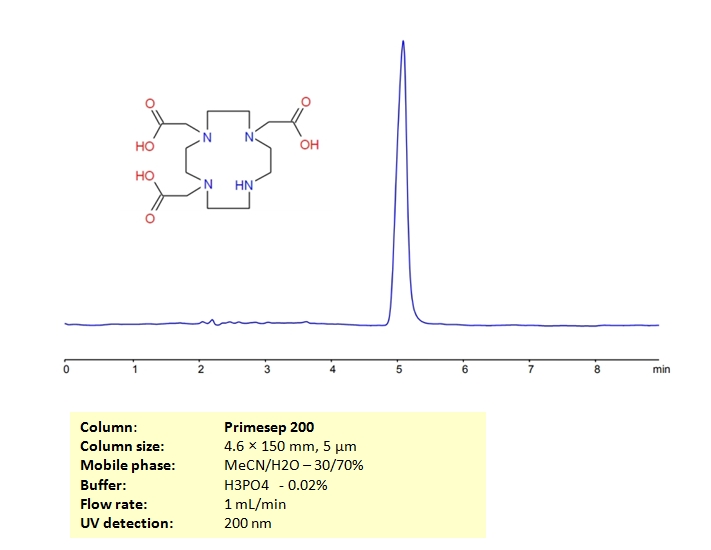
HPLC Determination of DO3A on Primesep 200 Column
June 18, 2019
HPLC Method for DO3A on Primesep 200 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of DO3A.
DO3A is a derrivative of DOTA and has the chemical formula C₁₄H₂₃N₄O₆Na₃. DOTA is an acronym for 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid. It is used to bind metal ions, partially due to it’s stability. It is often employed in biomedical applications including but not limited to MRI contrast agents and targeted cancer therapy.
DO3A can be retained and analyzed using the Primesep 200 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a [buffer] buffer. Detection is performed using UV.
| Column | Primesep 200 |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H2SO4 – 0.02 % |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable, Carboxylic acid, Drug, Carbocyclic. |
| Analyzing Compounds | DO3A |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended