| CAS Number | 106-51-4 |
|---|---|
| Molecular Formula | C6H4O2 |
| Molecular Weight | 108.097 |
| InChI Key | AZQWKYJCGOJGHM-UHFFFAOYSA-N |
| LogP | 0.2 |
| Synonyms |
|
Applications:
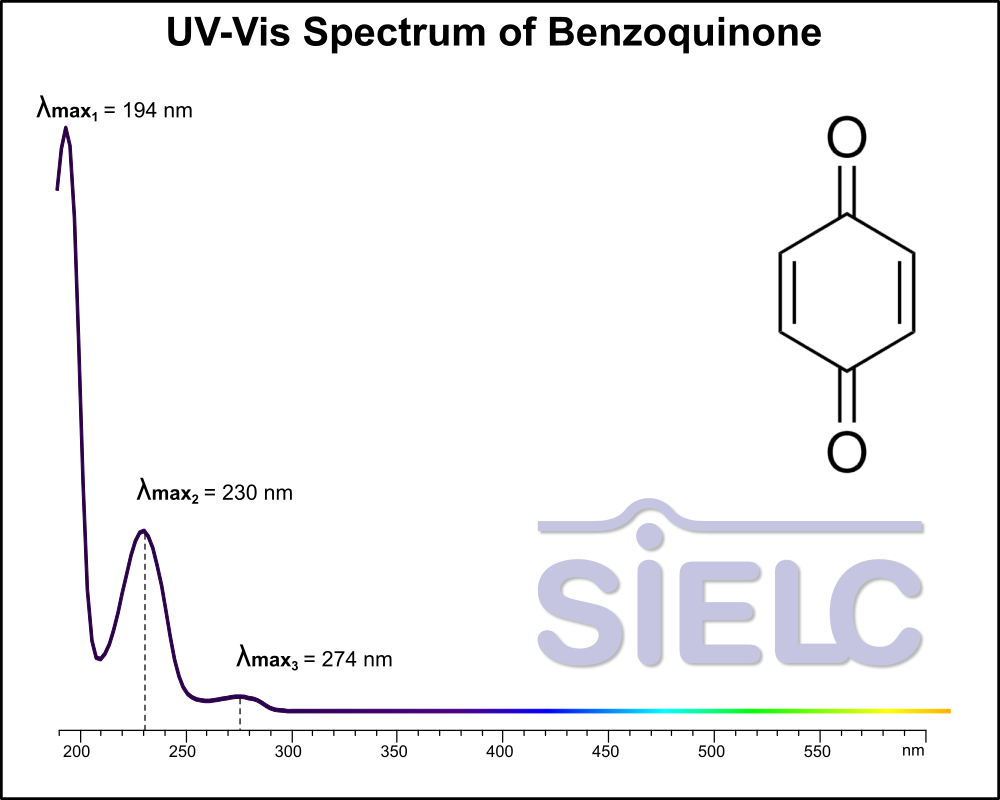
Uv-Vis Spectrum of Benzoquinone
January 23, 2026
If you are looking for optimized HPLC method to analyze Benzoquinone check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

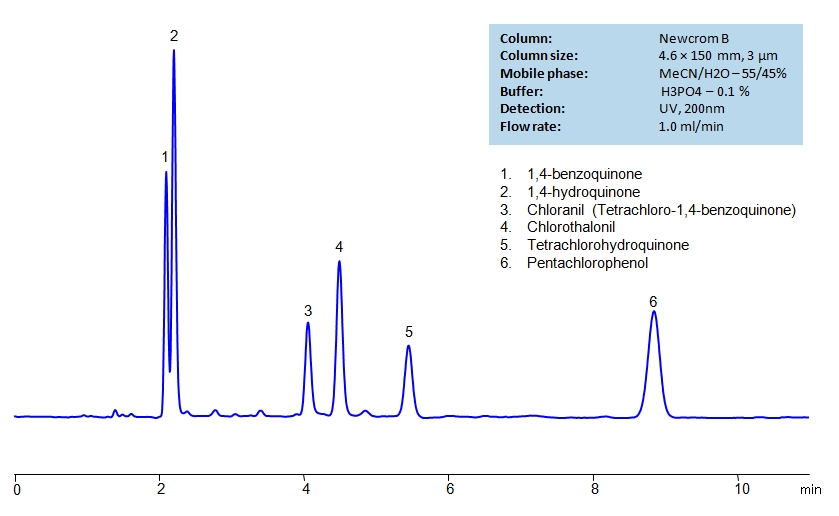
HPLC Separation of Haloaromatics and Quinones on Newcrom B Column
February 6, 2020
HPLC Method for Benzoquinone, Hydroquinone, Chlorothalonil, Chloranil, Pentachlorophenol, Tetrachlorohydroquinone on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Benzoquinone, Hydroquinone, Chlorothalonil, Chloranil, Pentachlorophenol, Tetrachlorohydroquinone.
1,4-benzoquinone, also known as para-quinone, is a compound with the formula C6H4O2. It is typically used as a precursor to hydroquinone, which is a reducing agent and antioxidant. It also serves as a dehydrogenation reagent and dienophile in Diels-Alder reactions.
1,4-hydroquinone is an aromatic derivative of benzene with the chemical formula C6H6O2. It is often used in skin whitening, although it has been banned by the United States Food and Drug Administration for over-the-counter use due to being a potential carcinogen. It can cause a variety of disease including but not limited to ochronosis. thyroid follicular cell hyperplasias, mononuclear cell leukemia, and adenomas. Agencies across the world encourage research into other agents to treat hyperpigmentation. You can find detailed UV spectra of hydroquinone and information about its various lambda maxima by visiting the following link.
Chloranil, also known as tetrachloro-1,4-benzoquinone, is a quinone with the chemical formula C6Cl4O2. It is a planar molecule that functions as an oxidant. It serves as a hydrogen acceptor and is more electrophilic than quinone. Chloranil is used to test for free secondary amines, which is useful to check for the presence of proline derivatives. Commercially, it is a precursor to dyes.
Chlorothalonil is a compound with a variety of uses as a fungicide, wood protectant, pesticide, and acaricide. It is used predominantly on peanuts, potatoes, and tomatoes. Outside of agriculture, it is also used in paints, resins, emulsions, and coatings. It’s chemical formula is C8Cl4N2.
Tetrachlorohydroquinone, also known as TCHQ, is a chlorinated organic compound with the chemical formula C6H2Cl4O2. It is a metabolote of the biocide pentachlorophenol. It causes damage to cells by increasing reactive oxygen species (ROS). It is harmful if swallowed and can cause serious eye damage.
Pentachlorophenol is a manufactured chemical with the chemical formula C6HCl5O. It is most often used as herbicide, insecticide, fungicide, algaecide, and disinfectant. Exposure to it can cause damage to liver, kidney, blood, lungs, eyes, skin, and mouth. It is classified as a probable human carcinogen.
Benzoquinone, Hydroquinone, Chlorothalonil, Chloranil, Pentachlorophenol, Tetrachlorohydroquinone can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a phosphoric acid buffer. Detection is performed using UV.
| Column | Newcrom B, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 55/45% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds | Haloaromatics, Quinones, Hydrophobic |
| Analyzing Compounds | Benzoquinone, Hydroquinone, Chlorothalonil, Chloranil, Pentachlorophenol, Tetrachlorohydroquinone |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Chloranil
Chlorothalonil
Hydroquinone
Pentachlorophenol
Tetrachlorohydroquinone

Dewetting Study in Aqueous Conditions
September 18, 2003
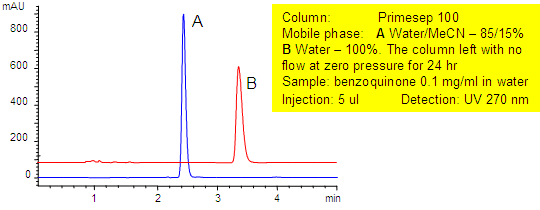
Most reversed-phase HPLC columns, such as C18, are incompatible with 100% aqueous mobile phases due to dewetting (collapse) of the stationary phase. Primesep columns combine reversed-phase with polar functional groups which eliminate the problems of dewetting. The Primesep 100 column in this application with an acetonitrile (MeCN, ACN) shows a retention time of 2.5 minutes. In 100% water, the retention time is 3 minutes even after sitting 24 hours in the water mobile phase. On a C18 phase, the analyte retention would have been lost and elution at the void (1.5 minutes) would have resulted.
| Column | Primesep 100 |
| Mobile Phase | MeCN/H2O |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options