| CAS Number | 88-99-3 |
|---|---|
| Molecular Formula | C8H6O4 |
| Molecular Weight | 166.133 |
| InChI Key | XNGIFLGASWRNHJ-UHFFFAOYSA-N |
| LogP | 0.73 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Phthalic acid on Primesep B Column
August 27, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Phthalic Acid on Primesep B by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Phthalic Acid
Phthalic acid is an aromatic dicarboxylic acid with the chemical formula C₆H₄(CO₂H)₂. It consists of a benzene ring with two carboxylic acid groups (-COOH) attached to adjacent carbon atoms (in the ortho position). It is mainly produced by the oxidation of naphthalene or o-xylene.
Uses
Phthalic acid is primarily used as a precursor to other chemicals, including:
Phthalic anhydride: Used in the production of plasticizers for PVC (polyvinyl chloride).
Alkyd resins: Important in the manufacture of paints and coatings.
Dyes and pigments: Phthalic acid derivatives are often used in the production of dyes.
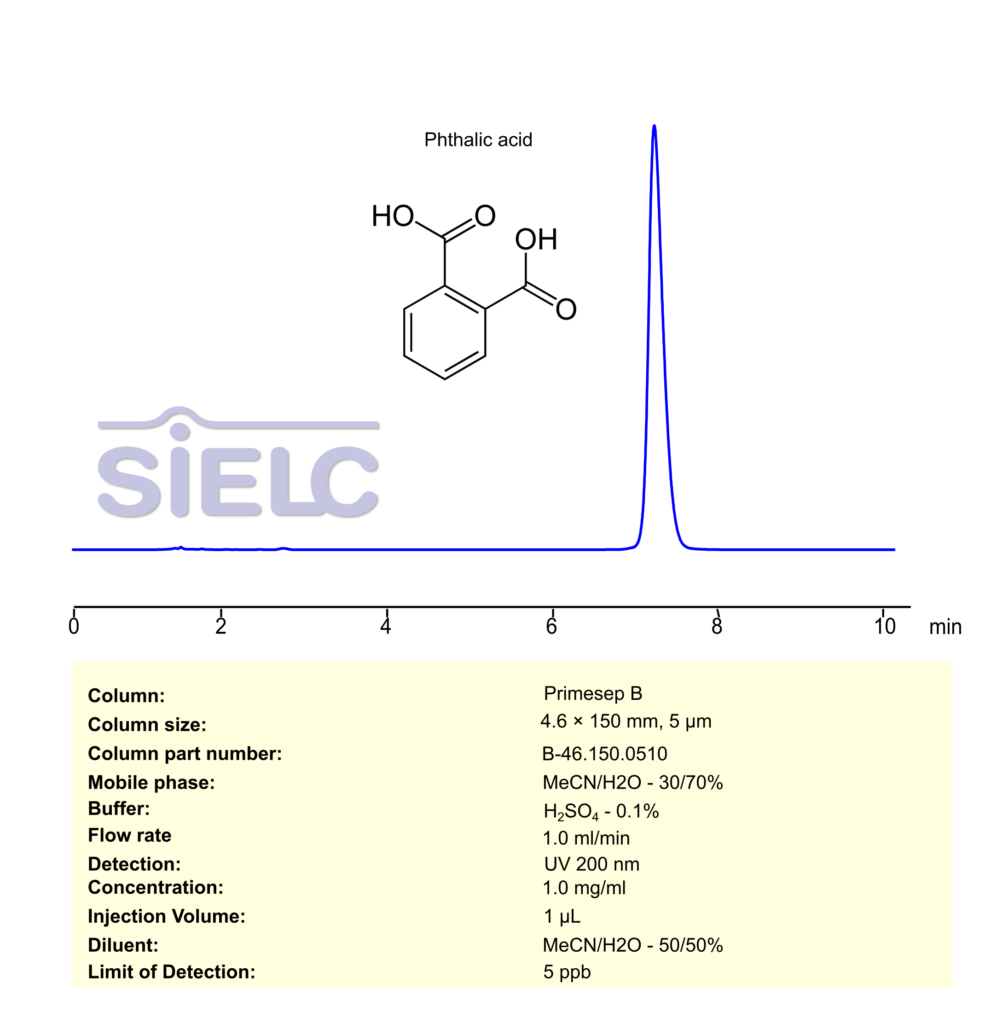
Phthalic Acid can be retained, separated and analyzed using a Primesep B mixed-mode stationary phase column. The analysis employs a gradient method with a simple mobile phase comprising water, acetonitrile (MeCN), and phosphoric acid as a buffer. This method allows for detection using UV 200 nm.
You can find detailed UV spectra of Phthalic Acid and information about its various lambda maxima by visiting the following link.
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Limit of Detection | 5 ppb |
| Class of Compounds | Acid |
| Analyzing Compounds | Phthalic Acid |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A

UV-Vis Spectrum of Phthalic acid
August 27, 2024
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

Separation of Phthalic Acids and Related Impurities
July 2, 2013
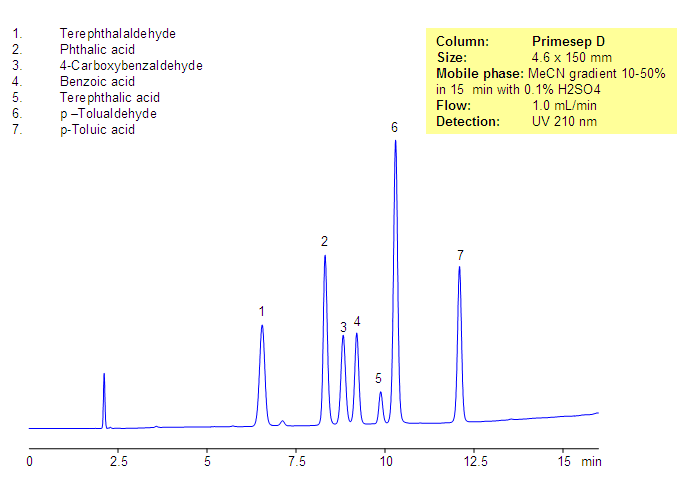
Phthalic acid, phthalic acid isomers, and related products present in the production of phthalic acid were separated on the Primesep D column, based on reversed-phase and in-exchange mechanisms. Neutral, hydrophobic compounds of the phthalic acid production are retained by a reversed-phase mechanism, and phthalic acid and other acidic compounds are retained by a combination of reversed-phase and anion-exchange mechanisms. Resolution and selectivity of this separation can be modified by varying the amount of acetonitrile, buffer concentrations, and buffer pH. This method can be used for monitoring the production cycle of phthalic acid and related impurities.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-50%, 15 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Terephthalaldehyde, Phthalic acid, 4-Carboxybenzaldehyde, Benzoic acid, Terephthalic acid, p –Tolualdehyde, p-Toluic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Phthalic Acid
Terephthalaldehyde
Terephthalic Acid
p-Tolualdehyde
p-Toluic Acid

HPLC Separation of Phthalic Acids using Hydrogen Bonding
June 18, 2012
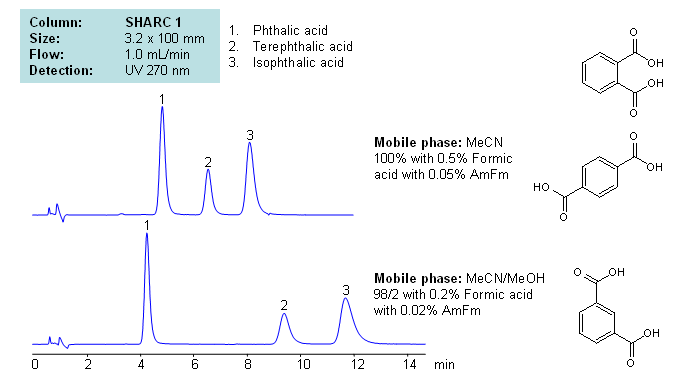
Phthalic acid, isophthalic acid and terephthalic acid are all isomers of each other. Being structurally similar, they can present difficulties to reverse-phase HPLC separation. Methods that require high organic concentrations in the mobile phase can cause dewetting in many reverse-phase columns. SHARC 1 column can be operated in anhydrous conditions and uses hydrogen bonding as the mechanism of separation. Here, phthalic acids were separated in pure acetonitrile (ACN), with the ability to adjust retention times by adding methanol (MeOH) to the mobile phase with formic acid and ammonium formate as buffer, making the method MS-compatible. Can also be UV detected at 270nm.
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Phthalic acid, Terephthalic acid, Isophthalic acid |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsPhthalic Acid
Terephthalic Acid

HPLC Separation of Organics Acids
November 21, 2006
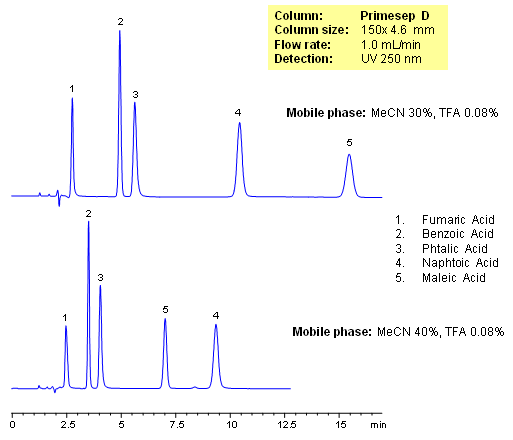
Primesep D separates organic acids such as fumaric, benzoic, phthalic, naphthoic, and maleic acids by a mixture of anion exchange and reversed phase. Retention times and elution order can be changed by adjusting the percentage of acetonitrile in the mobile. This can not be done by traditional ion-exchange and ion-exclusion chromatography. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) and UV detection at 250 nm.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Fumaric Acid, Benzoic Acid, Phthalic Acid, Maleic Acid, Naphtoic Acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsFumaric Acid
Maleic Acid
Naphthoic Acid
Organic Acids
Phthalic Acid

Separation of Diacid Hydrophobic and Ion Exchange Modes
October 11, 2005
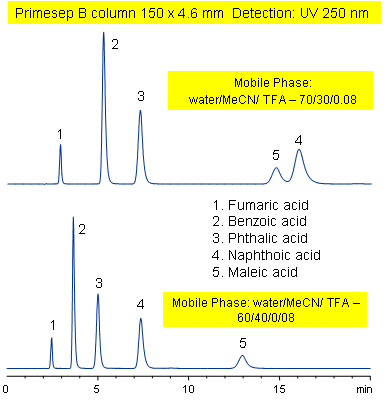
Primesep B combines a hydrophobic, reversed-phase mechanism with ion exchange to separate the diacids, fumaric, benzoic, phthalic, naphthoic, and maleic acids. Changing the acetonitrile content of the mobile phase reverses the peak order for naphthoic and maleic acids. Primesep B combines reversed-phase and anion-exchange mechanism with a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) and UV detection at 250 nm.
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Fumaric acid, Benzoic acid, Phthalic acid, Naphthoic acid, Maleic acid, ) |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDicarboxylic Acids
Fumaric Acid
Maleic Acid
Naphthoic Acid
Phthalic Acid