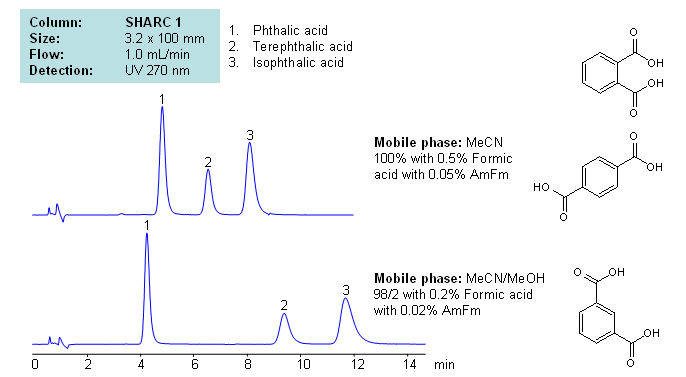
Phthalic acid, isophthalic acid and terephthalic acid are all isomers of each other. Being structurally similar, they can present difficulties to reverse-phase HPLC separation. Methods that require high organic concentrations in the mobile phase can cause dewetting in many reverse-phase columns. SHARC 1 column can be operated in anhydrous conditions and uses hydrogen bonding as the mechanism of separation. Here, phthalic acids were separated in pure acetonitrile (ACN), with the ability to adjust retention times by adding methanol (MeOH) to the mobile phase with formic acid and ammonium formate as buffer, making the method MS-compatible. Can also be UV detected at 270nm.
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Phthalic acid, Terephthalic acid, Isophthalic acid |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsPhthalic Acid
Terephthalic Acid





