| CAS Number | 137862-53-4 |
|---|---|
| Molecular Formula | C24H29N5O3 |
| Molecular Weight | 435.528 |
| InChI Key | ACWBQPMHZXGDFX-QFIPXVFZSA-N |
| LogP | 4.4 |
| Synonyms |
|
Applications:
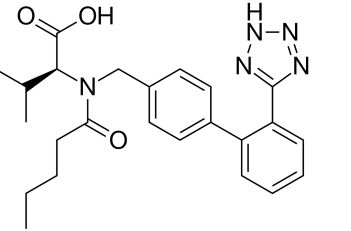
HPLC Method for Detection of NDEA in Valsartan Drug Substance
November 14, 2018
HPLC Method for Valsartan, N-Nitrosodiethylamine on Newcrom R1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Valsartan, N-Nitrosodiethylamine
Valsartan products are used to treat high blood pressure and congestive heart failure. On July 13, 2018, FDA announced a recall of valsartan tablets because of the potential for certain products to contain an impurity, N-nitrosodimethylamine (NDMA). Nitrosamine impurities is classified as a probable human carcinogen and is believed to have been introduced into the finished products as a result of the manufacturing process of the drug substance.
SIELC developed simple and fast HPLC method to measure presence of NDEA in drug formulations. This UV based method allows to detect ppb level of the compound in solution. Simple mobile phase comprised of acetonitrile (MeCN) and water with detection at 232 nm using Newcrom R1 column can be used to detect both the API and the NDEA.
| Column | Newcrom R1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-75%, 12 min |
| Buffer | H3PO4- 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 232 nm |
| Class of Compounds |
Drug, Carcinogen, Zwitterionic, Hydrophilic, Hydrophobic, Ionizable |
| Analyzing Compounds | Valsartan, N-Nitrosodiethylamine |
Application Column
Newcrom R1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Valsartan

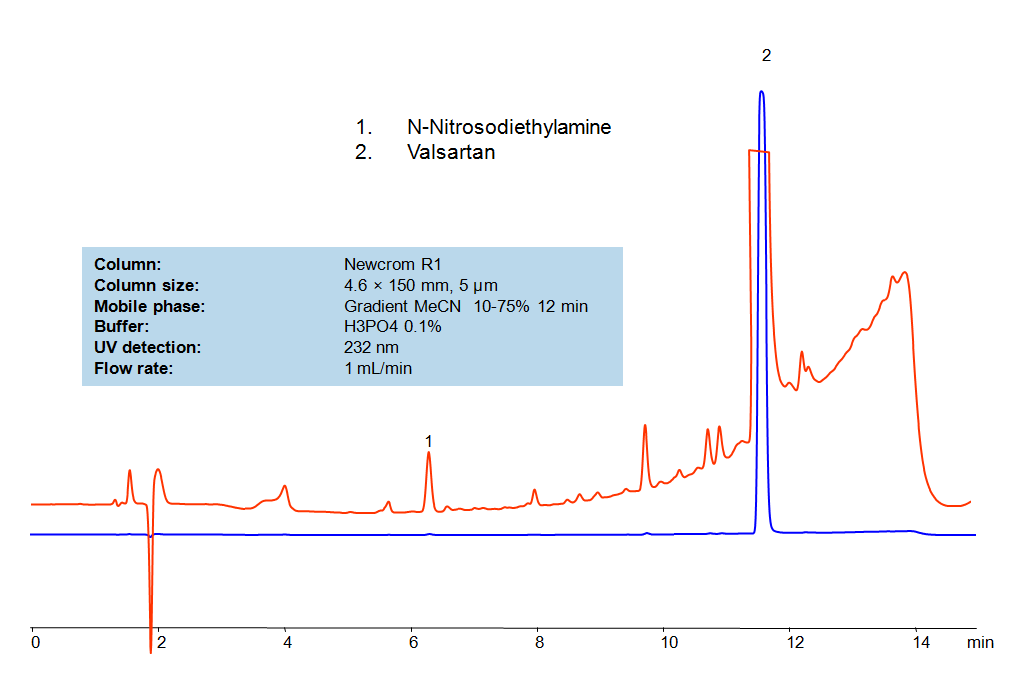
HPLC Method for Analysis of Valsartan
May 3, 2016
Valsartan is a nonpeptide triazole-derived antagonist of angiotensin that selectively and competitively blocks the binding of angiotensin II to the AT1 subtype receptor. It is used to treat high blood pressure and heart failure, as well as to improve the chance of someone living longer after a heart attack. Primesep 200, a reverse phase column, contains embedded acidic ionizable groups and can retain Valsartan. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
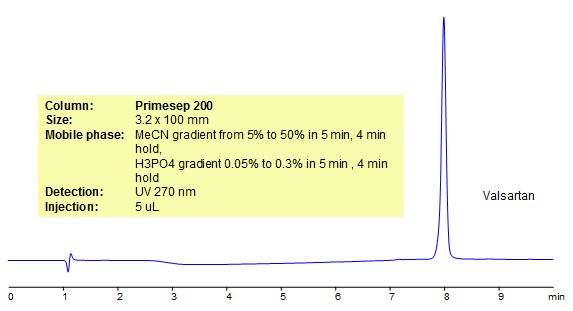
HPLC Analysis of Valsartan
August 6, 2015
Valsartan is a drug commonly used to treat high blood pressure and congestive heart failure. Legacy L1 was used to retain valsartan by reverse phase mechanism in this HPLC separation method. Legacy L1 uses embedded C18 groups on porous silica and is useful for many USP HPLC applications. Comparisons to Phenomenex columns are available by request. This simple HPLC method can be used in many pharmaceutical areas. For example, in batch production, impurity profiling, formulation and pharmacokinetics.
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 50% |
| Buffer | NaH2PO4 pH 2.5 – 20 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 254 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable |
| Analyzing Compounds | Valsartan |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options