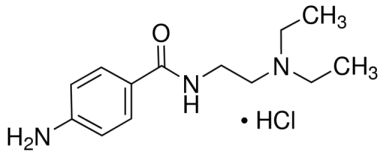
| CAS Number | 614-39-1 |
|---|---|
| Molecular Formula | C13H22ClN3O |
| Molecular Weight | 271.790 |
| InChI Key | ABTXGJFUQRCPNH-UHFFFAOYSA-N |
| LogP | 0.994 |
| Synonyms |
|
Applications:
USP Methods Analysis of Procainamide on Legacy L1 Column
June 21, 2012
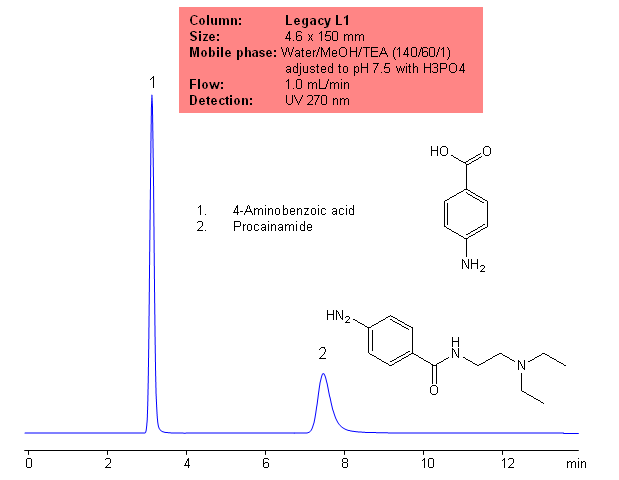
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Procainamide and 4-aminobenzoic acid
Mobile phase: WAter/MeOH/MeCN (70/70/90) with 7 mM sodium lauryl sulfate and 11mM phosphoric acid
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Water/MeOH/TEA (140/60/1) adjusted to pH 7.5 with H3PO4 |
| Buffer | TEAPh |
| Flow Rate | 1.5 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Nonsteroidal anti-inflammatory drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Ibuprofen, benzophenone |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsProcainamide
Procainamide Hydrochloride