| CAS Number | 53-03-2 |
|---|---|
| Molecular Formula | C21H26O5 |
| Molecular Weight | 358.434 |
| InChI Key | XOFYZVNMUHMLCC-ZPOLXVRWSA-N |
| LogP | 1.46 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Prednisone
July 25, 2024
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

Alltesta HPLC Method for Separating Corticosteroids such as Prednisone, Prednisolone and Methylprednisolone on Primesep B Column
May 30, 2024
Alltesta HPLC Method for Prednisone, Methylprednisolone, Prednisolone on Primesep B by SIELC Technologies
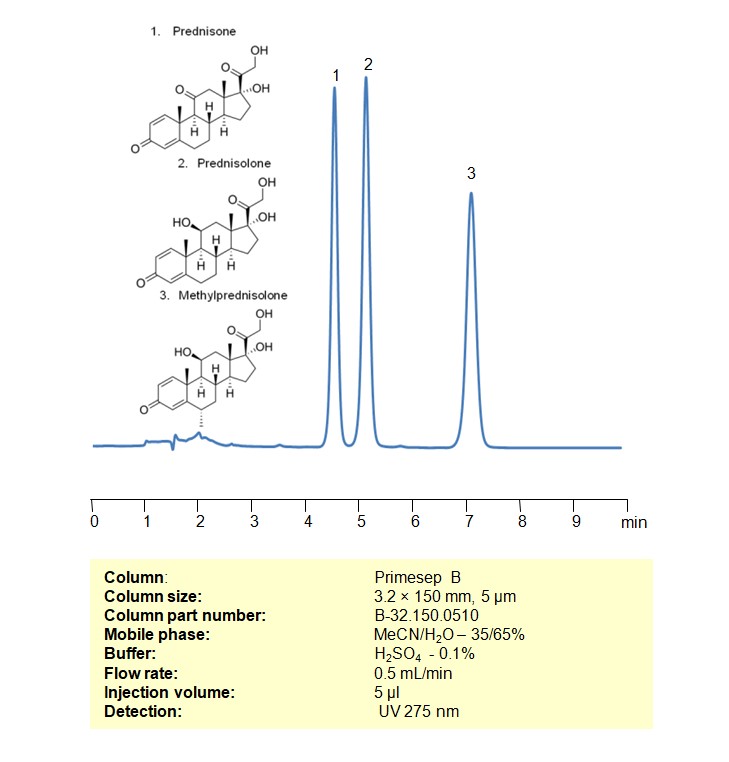
High Performance Liquid Chromatography (HPLC) Method for Analysis of Prednisone, Methylprednisolone, Prednisolone
All three compounds have potent anti-inflammatory effects, making them valuable in treating conditions where inflammation plays a role.
Prednisone:
- Mechanism of Action: Prednisone is a synthetic corticosteroid that mimics the action of cortisol, a natural steroid produced by the adrenal glands. It primarily exerts its effects by binding to glucocorticoid receptors and regulating gene expression.
- Medical Uses: It is commonly used to treat inflammatory conditions, autoimmune disorders, allergic reactions, and certain cancers.
Prednisolone:
- Derived Form: Prednisolone is the active form of prednisone. When prednisone is ingested, the liver converts it into prednisolone.
- Mechanism of Action: Similar to prednisone, it has anti-inflammatory and immunosuppressive properties.
Methylprednisolone:
- Potency: Methylprednisolone is considered to be more potent than prednisone.
- Medical Uses: It is used in various conditions, including severe allergic reactions, inflammatory conditions, and as a part of immunosuppressive regimens.
Corticosteroids can be retained, separated, and analyzed using a Primesep B mixed-mode stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer. Detection is achieved using UV at 245 nm
| Column | Primesep B, 3.2 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | H2SO4 -0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Corticosteroids |
| Analyzing Compounds | Prednisone, Methylprednisolone, Prednisolone |
Application Column
Primesep B
Column Diameter: 3.2 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Prednisolone
Prednisone

HPLC Method for Analysis of Prednisone on Primesep B Column
January 29, 2024
HPLC Method for Analysis of Prednisone on Primesep B by SIELC Technologies
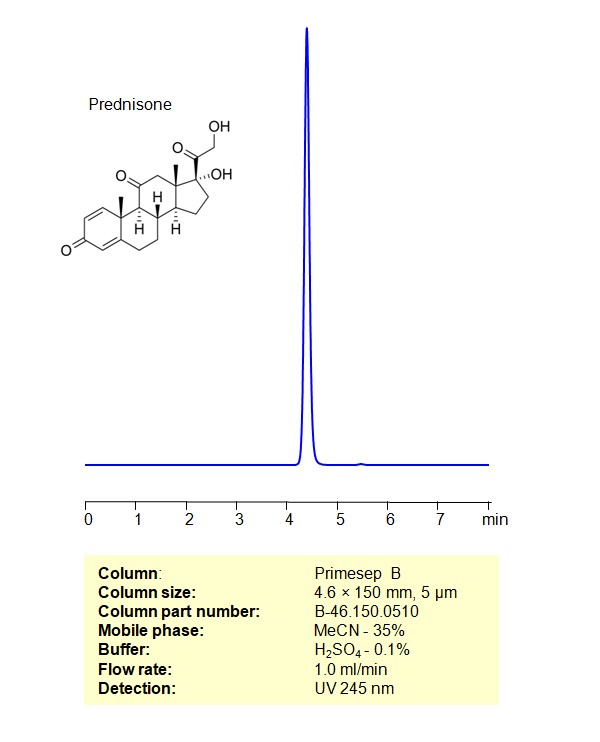
High Performance Liquid Chromatography (HPLC) Method for Analysis of Prednisone
Prednisone is a synthetic corticosteroid drug that belongs to the class of glucocorticoids. It is a prodrug, meaning that it is biologically inactive until it undergoes metabolic activation in the liver, where it is converted into its active form, prednisolone. Both prednisone and prednisolone have anti-inflammatory and immunosuppressant properties and are used for various medical conditions.
- Anti-inflammatory: Prednisone is used to reduce inflammation in various conditions such as arthritis, allergic reactions, and inflammatory bowel diseases.
- Immunosuppression: It is employed to suppress the immune system in conditions like organ transplantation and certain autoimmune diseases.
- Respiratory Conditions: Prednisone is often prescribed for respiratory conditions like asthma and chronic obstructive pulmonary disease (COPD).
Prednisone can be retained, and analyzed using a Primesep B mixed-mode stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer. Detection is achieved using UV 245 nm
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 245 nm |
| Class of Compounds | Corticosteroids |
| Analyzing Compounds | Prednisone |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method for Separating Corticosteroids such as Prednisone, Prednisolone and Methylprednisolone on Primesep B Column
January 29, 2024
HPLC Method for Prednisone, Prednisolone, Methylprednisolone on Primesep B by SIELC Technologies
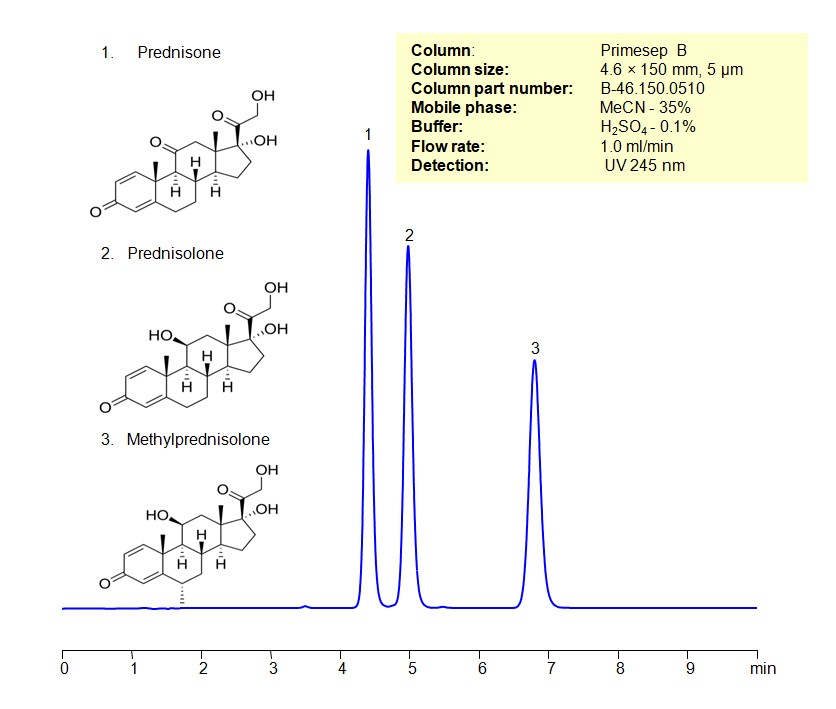
High Performance Liquid Chromatography (HPLC) Method for Analysis of Prednisone, Prednisolone, Methylprednisolone
All three compounds have potent anti-inflammatory effects, making them valuable in treating conditions where inflammation plays a role.
Prednisone:
- Mechanism of Action: Prednisone is a synthetic corticosteroid that mimics the action of cortisol, a natural steroid produced by the adrenal glands. It primarily exerts its effects by binding to glucocorticoid receptors and regulating gene expression.
- Medical Uses: It is commonly used to treat inflammatory conditions, autoimmune disorders, allergic reactions, and certain cancers.
Prednisolone:
- Derived Form: Prednisolone is the active form of prednisone. When prednisone is ingested, the liver converts it into prednisolone.
- Mechanism of Action: Similar to prednisone, it has anti-inflammatory and immunosuppressive properties.
Methylprednisolone:
- Potency: Methylprednisolone is considered to be more potent than prednisone.
- Medical Uses: It is used in various conditions, including severe allergic reactions, inflammatory conditions, and as a part of immunosuppressive regimens.
Corticosteroids can be retained, separated, and analyzed using a Primesep B mixed-mode stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer. Detection is achieved using UV at 245 nm
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | H2SO4-0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 245 nm |
| Class of Compounds | Corticosteroids |
| Analyzing Compounds | Prednisone, Prednisolone, Methylprednisolone |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Prednisolone
Prednisone

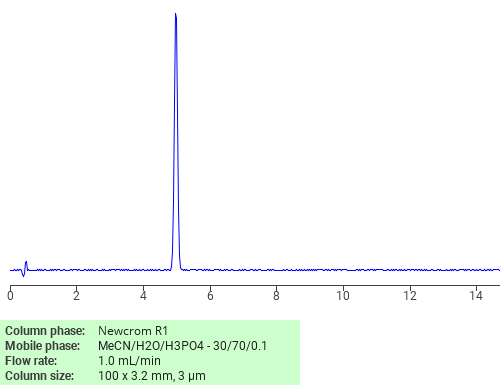
Separation of Prednisone on Newcrom R1 HPLC column
February 16, 2018
Prednisone can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options