| CAS Number | 68-12-2 |
|---|---|
| Molecular Formula | HCON(CH3)2 |
| Molecular Weight | 73.09 |
| InChI Key | ZMXDDKWLCZADIW-UHFFFAOYSA-N |
| LogP | -1 |
| Synonyms |
|
Applications:
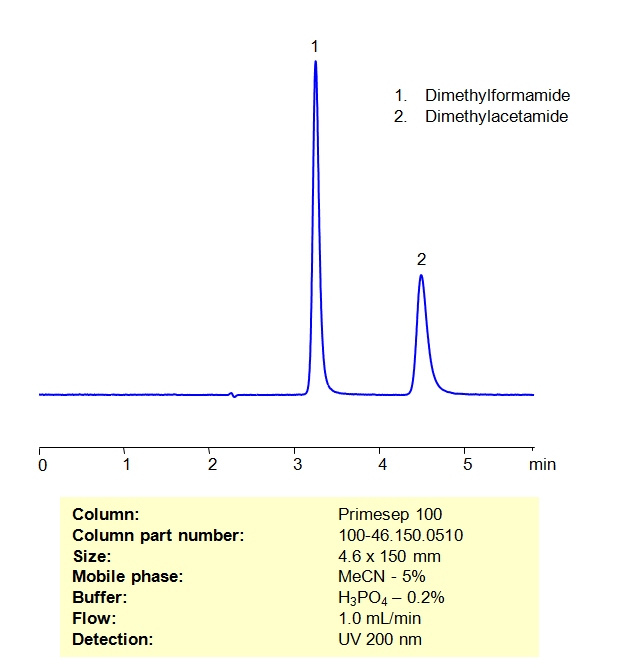
HPLC Method For Analysis Of Dimethylformamide and Dimethylacetamide on Primesep 100 Column
April 12, 2022
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Dimethylformamide and Dimethylacetamide
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | H3PO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm, |
| Class of Compounds |
Amide |
| Analyzing Compounds | TDimethylformamide, Dimethylacetamide |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDMF (Dimethylformamide)

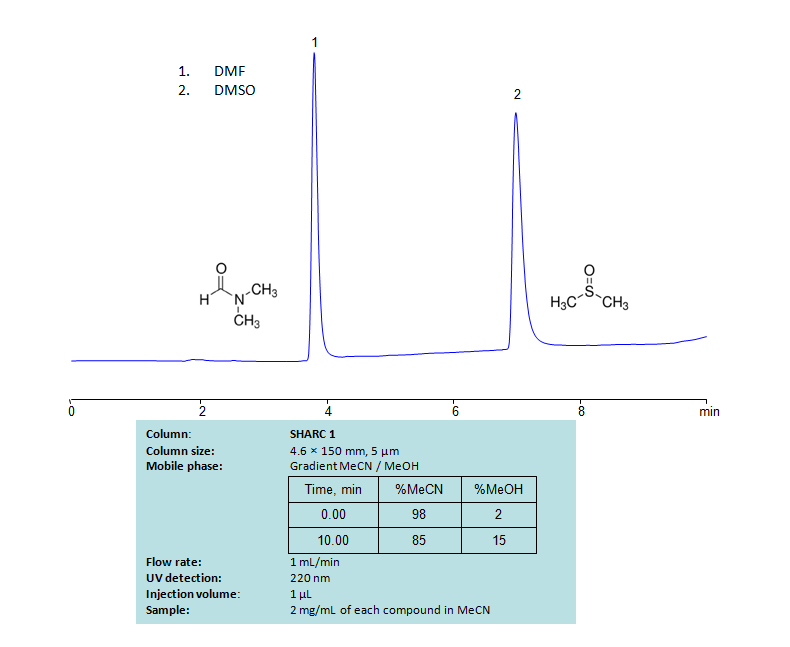
HPLC Separation of Mixture of DMF and DMSO
April 25, 2019
HPLC Method for DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide) on SHARC 1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide).
Dimethylformamide, DMF, is primarily used as an organic solvent and is miscible with water and most other organic liquids. Dimethyl sulfoxide (DMSO) is also a solvent. It can dissolve both polar and nonpolar compounds and is miscible with water and other organic liquids. Both compounds can be retained and separated using anhydrous (water-free) conditions using HPLC by SHARC 1 column, which uses hydrogen-bonding as a separation mechanism. The method uses a gradient of acetonitrile (ACN) and methanol (MeOH) mobile phase without the need for a buffer. UV detection used at 220nm.
| Column | SHARC 1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 98-85 %, 10 min |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 220 nm |
| Class of Compounds |
Drug, Basic, Hydrophobic, Ionizable, Zwitterionic |
| Analyzing Compounds | DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide) |
Application Column
SHARC 1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
DMSO (Dimethyl sulfoxide)