| CAS Number | 91-64-5 |
|---|---|
| Molecular Formula | C9H6O2 |
| Molecular Weight | 146.146 |
| InChI Key | ZYGHJZDHTFUPRJ-UHFFFAOYSA-N |
| LogP | 1.39 |
| Synonyms |
|
Applications:
Uv-Vis Spectrum of Coumarin
February 20, 2026
Access the UV-Vis Spectrum SIELC Library

If you are looking for optimized HPLC method to analyze Coumarin check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of Coumarin
July 18, 2018
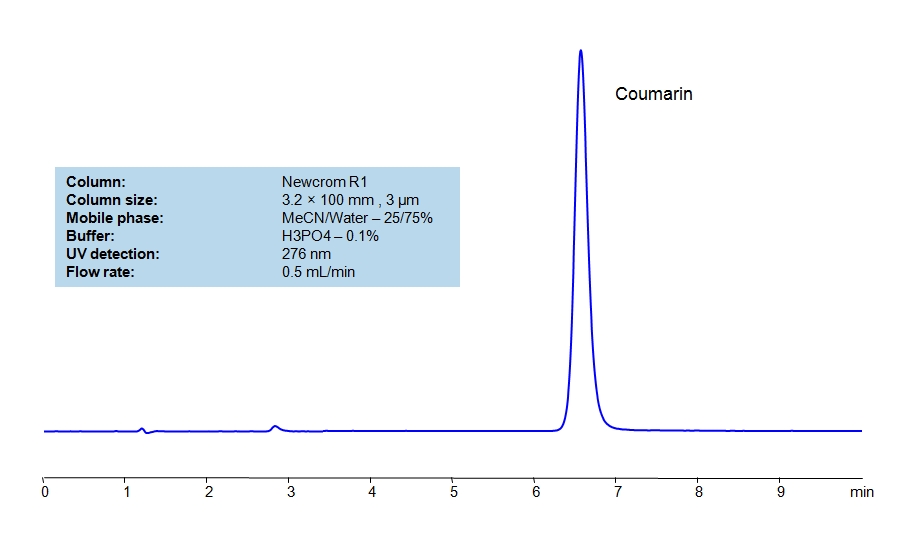
HPLC Method for Coumarin on Newcrom R1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Coumarin
Coumarin is an aromatic chemical that is used as a fragrance ingredient. It has a sweet earthy scent and is typically found in Tonka beans, cinnamon, woodruff, bison grass, and green tea. Besides a pleasant smell it had anti-inflammatory, antibacterial, and antifungal properties.
Coumarin an be retained and analyzed using the Newcrom R1 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a phosphoric acid buffer. Detection is performed using UV.
| Column | Newcrom R1, 3.2 x 100 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | H3PO4 – 0.1 % |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 276 nm |
| Class of Compounds | Drug, Flavouring substance, Hydrophobic, Neutral |
| Analyzing Compounds | Coumarin |
Application Column
Newcrom R1
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended