| CAS Number | 81-07-2 |
|---|---|
| Molecular Formula | C7H5NO3S |
| Molecular Weight | 183.180 |
| InChI Key | CVHZOJJKTDOEJC-UHFFFAOYSA-N |
| LogP | 0.91 |
| Synonyms |
|
Applications:
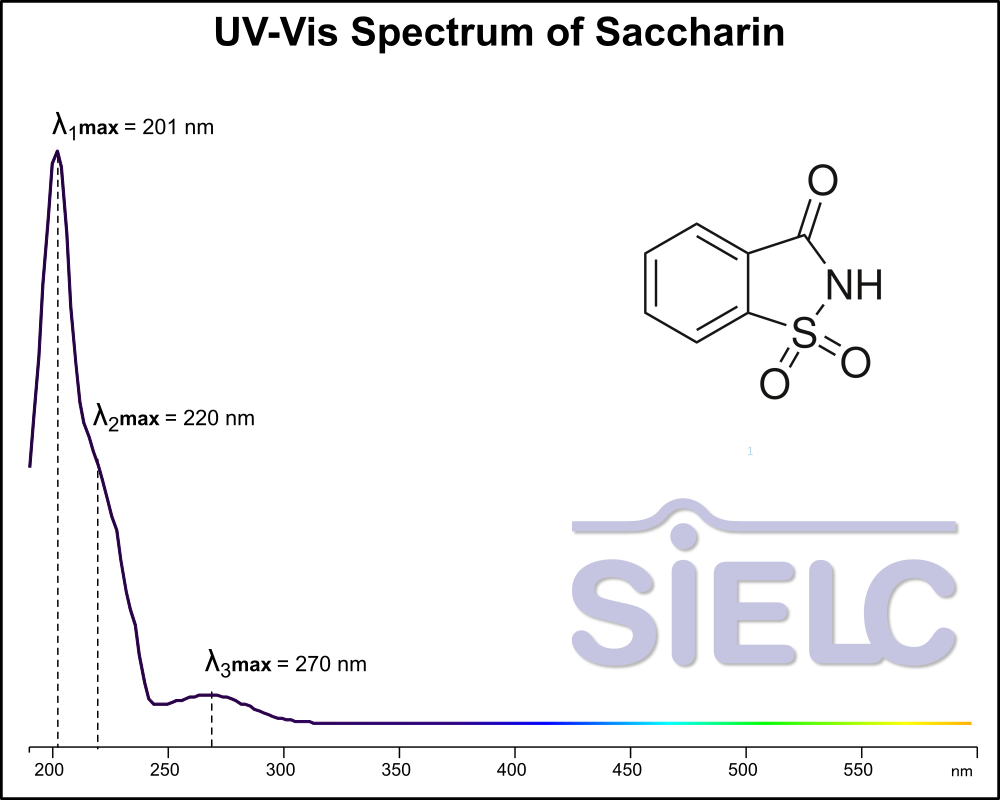
Uv-Vis Spectrum of Saccharin
February 3, 2026
If you are looking for optimized HPLC method to analyze Saccharin check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Separation of Saccharin and Sorbitol
October 14, 2010
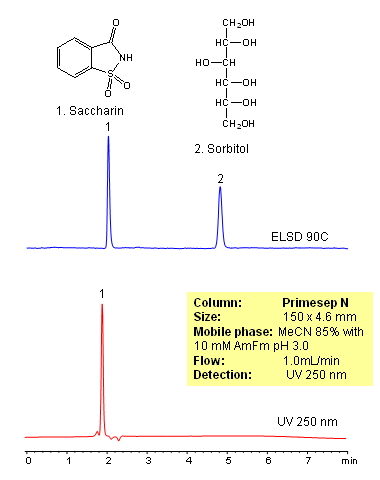
Sorbitol and saccharin are two sugar substitutes used in food and drinks. Separation of sorbitol and saccharin was achieved on a Primesep N HILIC column using acetonitrile/water with ammonium formate. Compounds were monitored by combination of UV and ELSD. Method is compatible with LC/MS detection.
Application Column
Primesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSorbitol

Separation of Alginic Acid and Related Products
August 22, 2008
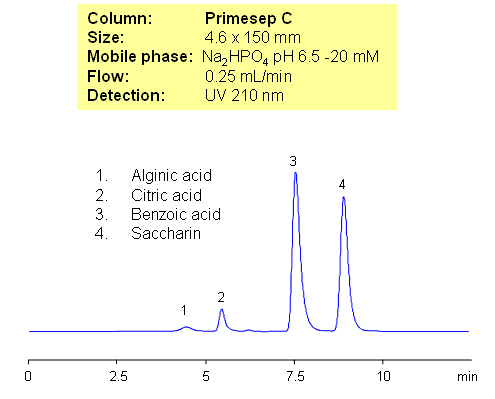
Alginate is used in various pharmaceutical preparations. Chemically, it is a linear copolymer with homopolymeric blocks of (1-4)-linked ?-D-mannuronate (M) and its C-5 epimer ?-L-guluronate (G) residues, respectively, covalently linked together in different sequences or blocks. Alginic acid can be separated from benzoate, citric acid and saccharin by mixed-mode chromatography on Primesep C HPLC column. This method can be used to quantitate alginic acid, citric acid or saccharin in complex mixtures. Various detection technique can be used (UV, ELSD, LC/MS), based on mobile phase selection.
| Column | Primesep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Na2HPO4 |
| Flow Rate | 0.25 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Alginic acid, Citric acid, Benzoic acid, Saccharin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Citric Acid
Saccharin